Knowing the units of the rate constant is important as it is used often for solving problems related to the rate laws.
k Units of a Zero-Order Reaction
Zero-order indicates that the rate does not depend on the concentration, and therefore, the rate is equal to the concentration.
rate = k[A]0
[A]0 = 1, therefore,
rate = k
The units for the rate are mol/L, so it is the same as the rate constant:
k = mol/L s or M/s or M x s-1
k Units of a First-Order Reaction
Let’s assume it is a first-order reaction in molecule A:
rate = k[A]
The units for the rate are mol/L. The rate constant is equal to:
\[k\; = \;\frac{{{\rm{rate}}}}{{\left[ {\rm{A}} \right]}}\]
And now, add the units for the rate and concentration:
\[k\; = \;\frac{{{\rm{mol}}}}{{{\rm{L}}\; \times \;{\rm{s}}}}\; \div \;\frac{{{\rm{mol}}}}{{{\rm{L}}\;}}\; = \;\frac{{\cancel{{{\rm{mol}}}}}}{{\cancel{{\rm{L}}}\; \times \;{\rm{s}}}}\; \times \;\frac{{\cancel{{\rm{L}}}}}{{\cancel{{{\rm{mol}}}}}}\; = \;{{\rm{s}}^{{\rm{ – 1}}}}\]
k Units of a Second-Order Reaction
Let’s assume it is a second-order reaction in molecule A:
rate = k[A]2
\[k\; = \;\frac{{{\rm{rate}}}}{{{{\left[ {\rm{A}} \right]}^2}}}\]
And now, add the units for the rate and concentration:
\[k\; = \;\frac{{{\rm{mol}}}}{{{\rm{L}}\; \times \;{\rm{s}}}}\; \div \;{\left( {\frac{{{\rm{mol}}}}{{{\rm{L}}\;}}} \right)^2}\; = \;\frac{{\cancel{{{\rm{mol}}}}}}{{\cancel{{\rm{L}}}\;{\rm{ \times }}\;{\rm{s}}}}\;{\rm{ \times }}\;\frac{{{{\rm{L}}^{\cancel{{\rm{2}}}}}}}{{{\rm{mol}}\cancel{{^{\rm{2}}}}}}\;\;{\rm{ = }}\;\frac{{\rm{L}}}{{{\rm{mol}}\; \times \;{\rm{s}}}}\]
Alternatively, this can be written as M-1 · s-1
A Shortcut to Determining the Units of Rate Constant
Notice how for each order, we determined the units of k by dividing the rate by molarity raised to the power of the reaction order:
\[k\; = \;\frac{{{\rm{rate}}}}{{{{\left[ {\rm{A}} \right]}^n}}}\]
where n is the reaction order
From this equation, a general formula for the units of k is obtained which is:
k units = M1-n · t-1
where n is the reaction order
For example, let’s say we want to determine the units of the rate constant for third-order reactions.
n = 3, and therefore,
k units = M1-3 · t-1 = M-2 · t-1
If the time is seconds, then the units will be:
k units = M-2 · s-1
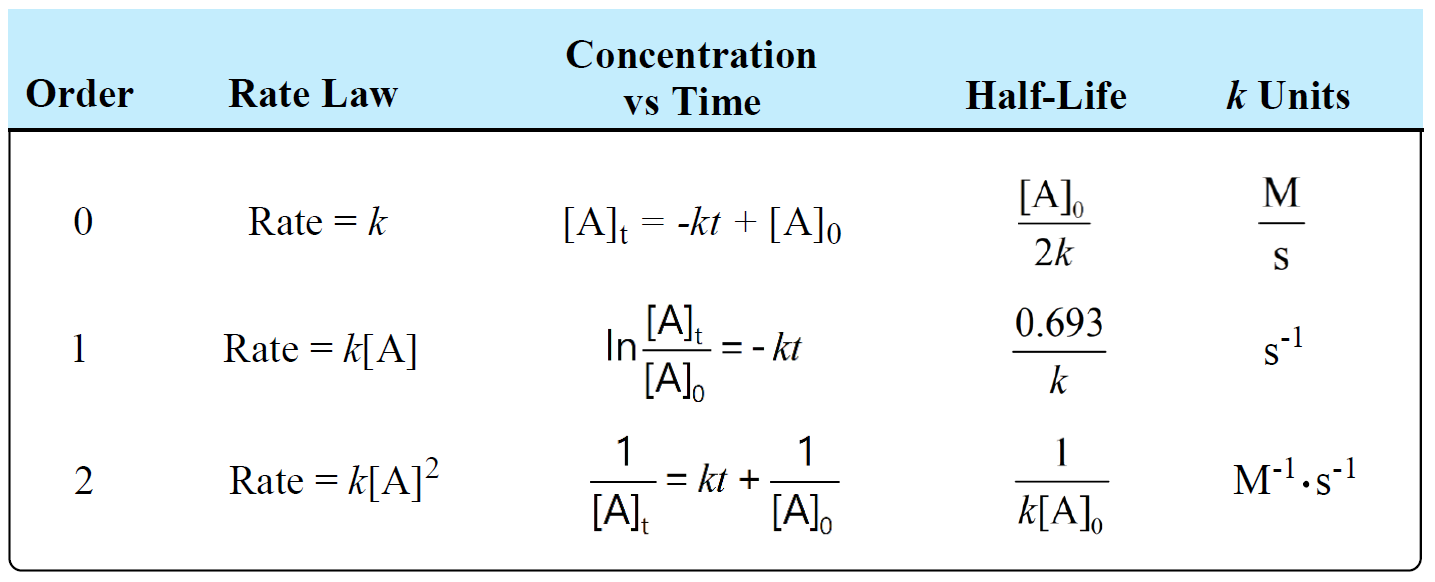
The following table summarizes the rate laws, half-lives, and k units for first-, second-, and zero-order reactions:
Here is a 77-question, Multiple-Choice Quiz on Chemical Kinetics:
Check Also
- Reaction Rate
- Rate Law and Reaction Order
- How to Determine the Reaction Order
- Integrated Rate Law
- The Half-Life of a Reaction
- Determining the Reaction Order Using Graphs
- How Are Integrated Rate Laws Obtained
- Activation Energy
- The Arrhenius Equation
- Chemical Kinetics Practice Problems

