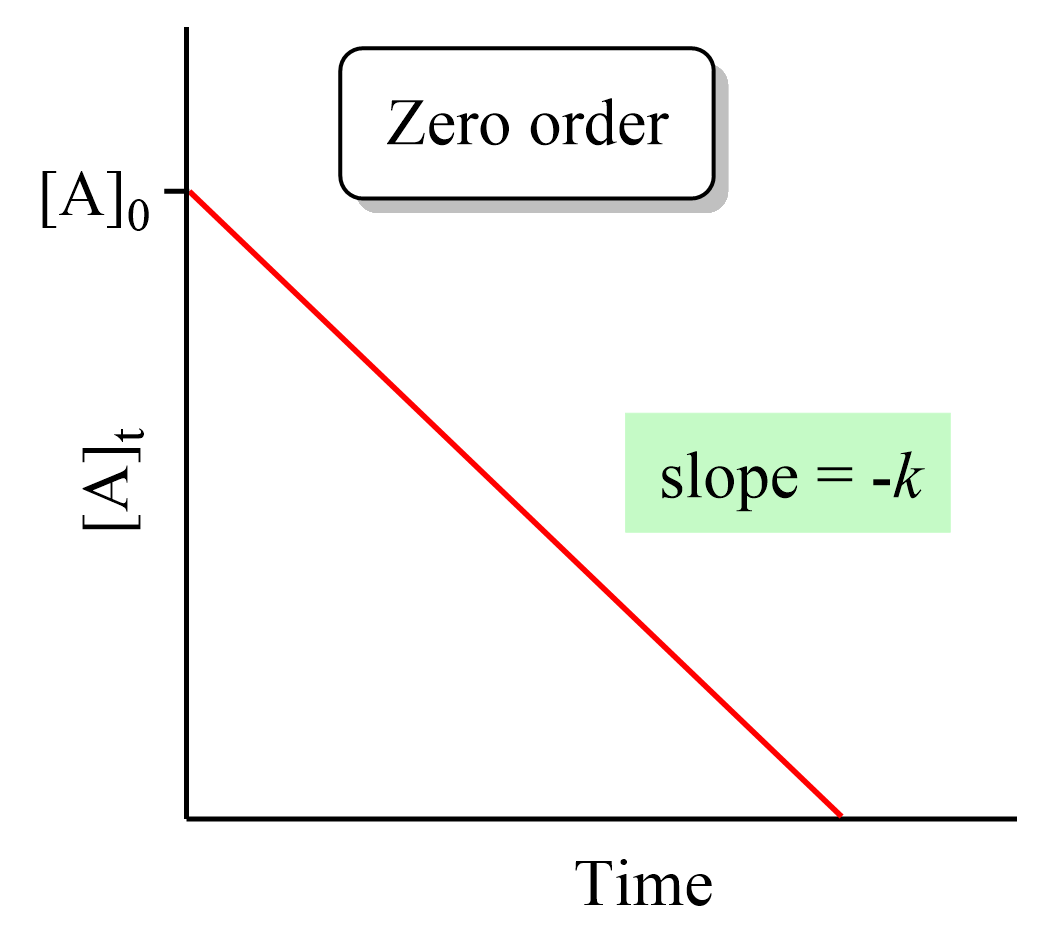
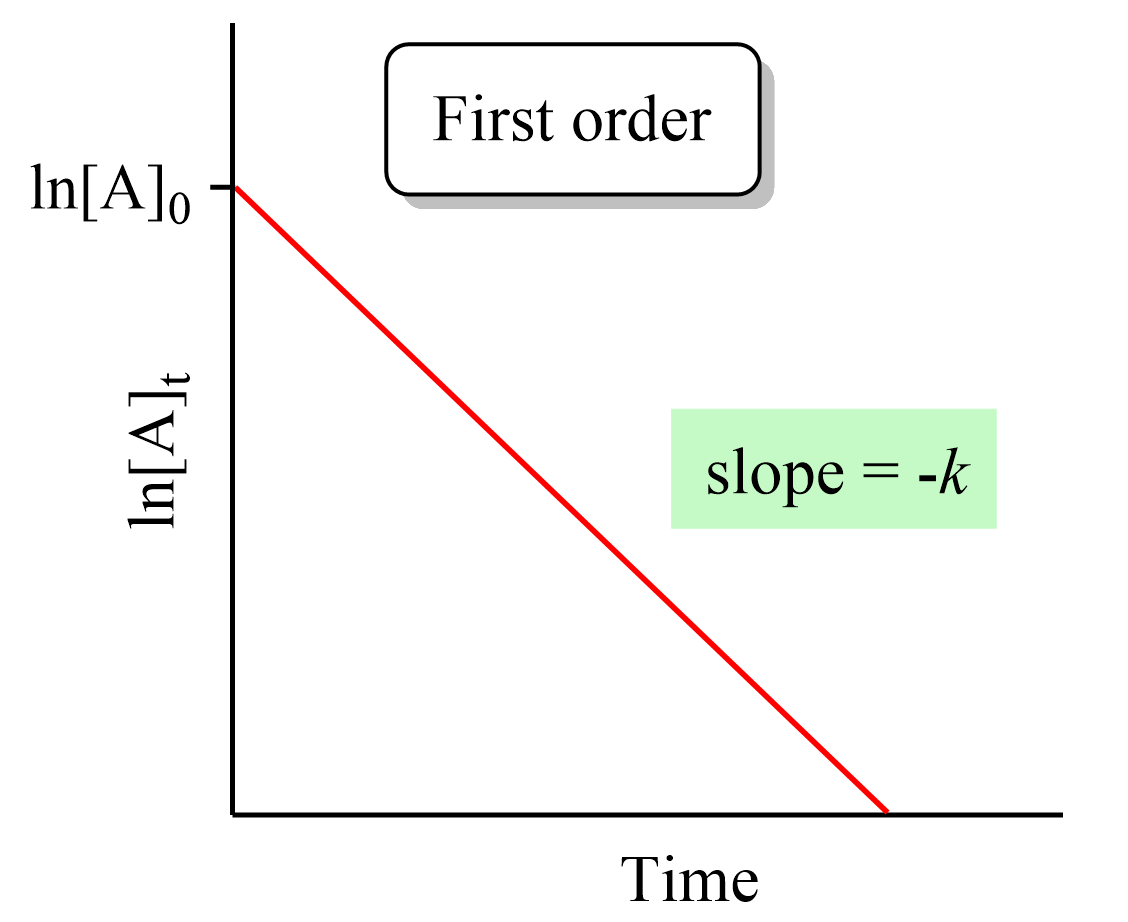
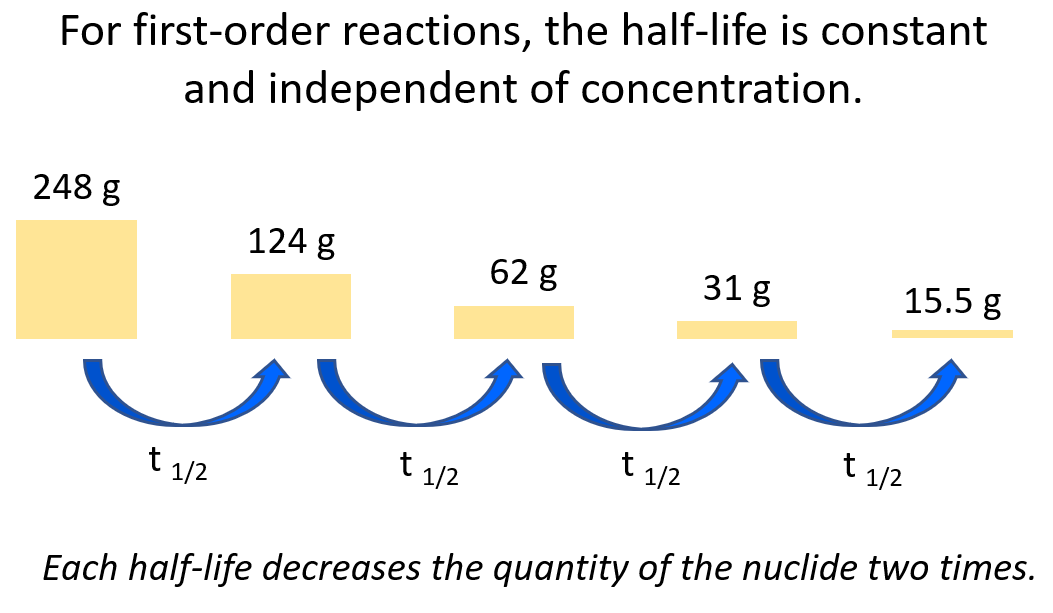
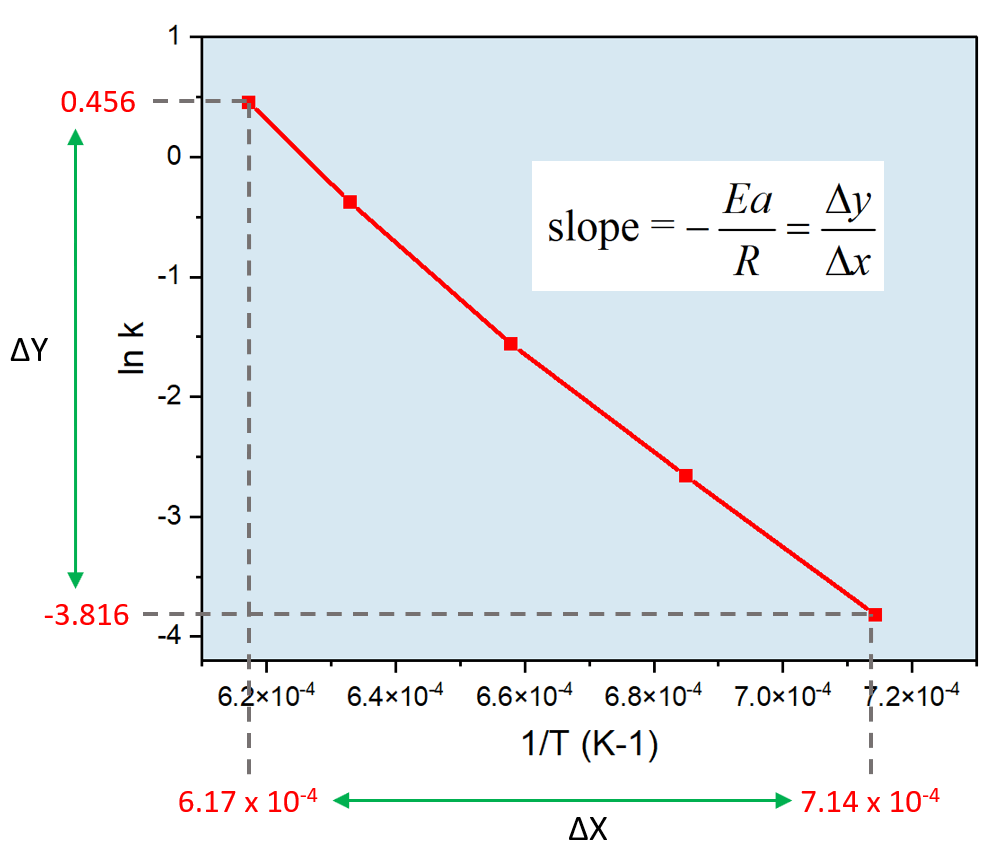
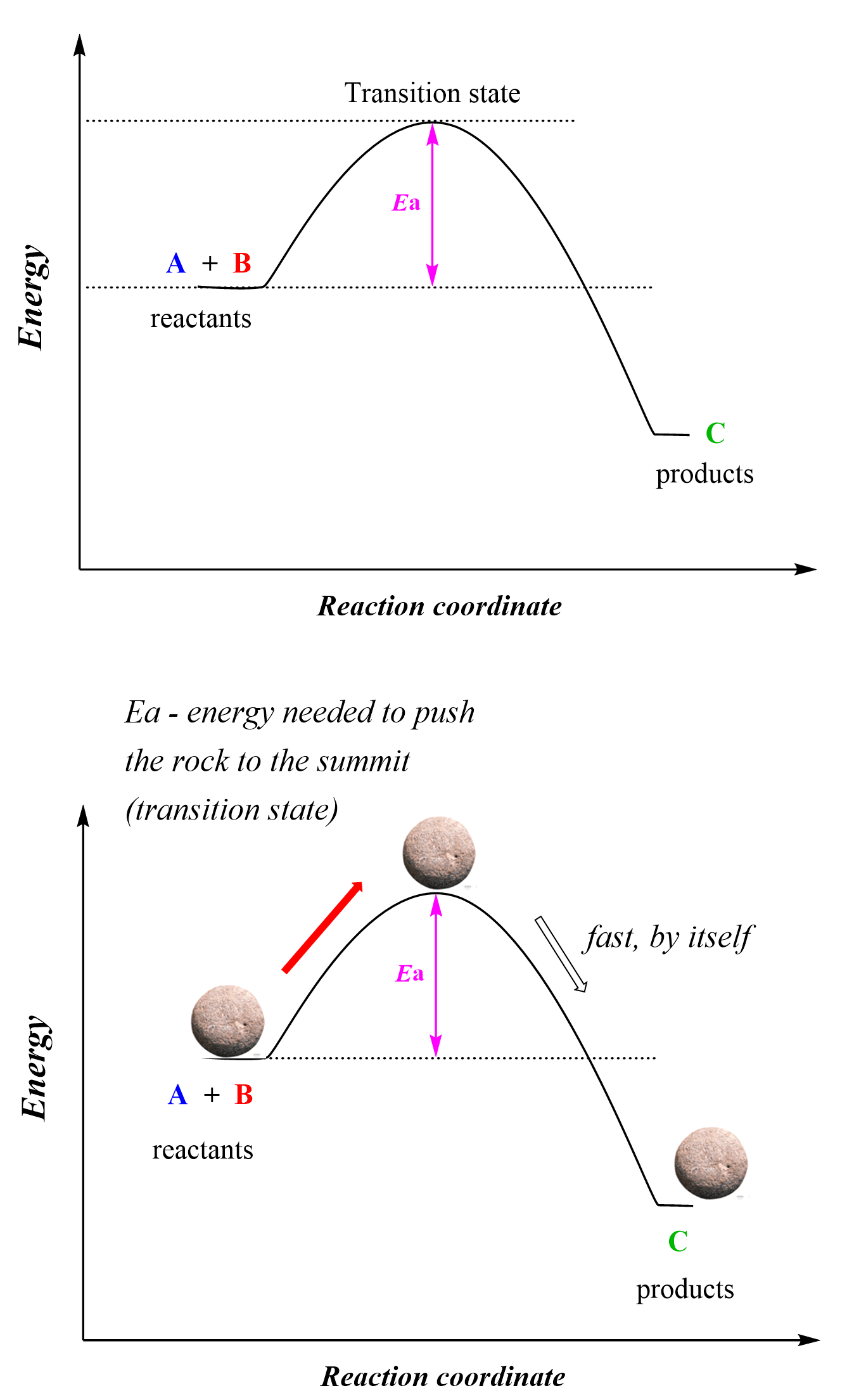
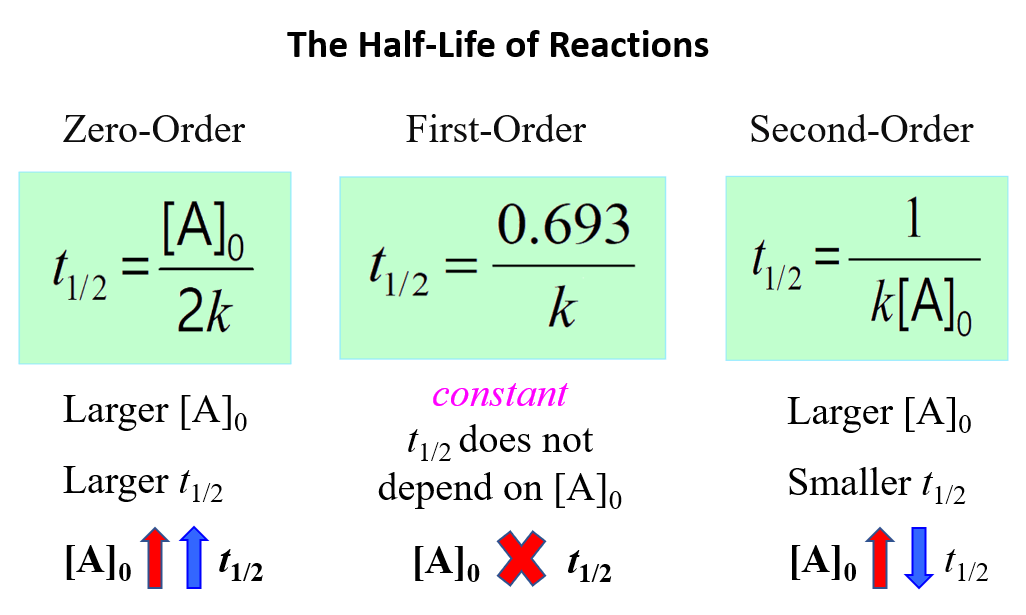
Zero-Order Reactions
In a zero-order reaction, the rate of the reaction is independent of the concentration of the reactant. This can be seen in the differential rate law which shows how the rate of a reaction depends on the concentration of the … Read more