In the previous post, we talked about the hybridization of atomic orbitals, so this practice set is to master to concepts of determining whether an atom is sp, sp2, sp3, sp3d, or sp3d2 hybridized.
Practice
Determine the hybridization, the electron- and molecular geometry of the following molecules.
| (a) BF3 | (b) CH2O | (c) HCN | (d) BeCl2 | (e) CH2Cl2 |
| (f) SOCl2 | (g) SO2 | (h) PCl5 | (i) XeO4 | (j) NCl3 |
| (k) SiCl4 | (l) SF2 | (m) H2S | (n) SO3 | (o) COCl2 |
| (p) PCl3 | (q) OF2 | (r) BrF5 | (s) N2O | (t) SF6 |
| (u) POCl3 | (x) XeF2 | (y) XeF4 | (z) C2H2 |
For each marked atom, add any missing lone pairs of electrons to determine the steric number, electron and molecular geometry, approximate bond angles, and the hybridization state.
You can also download the questions as a PDF worksheet to print and work on here.
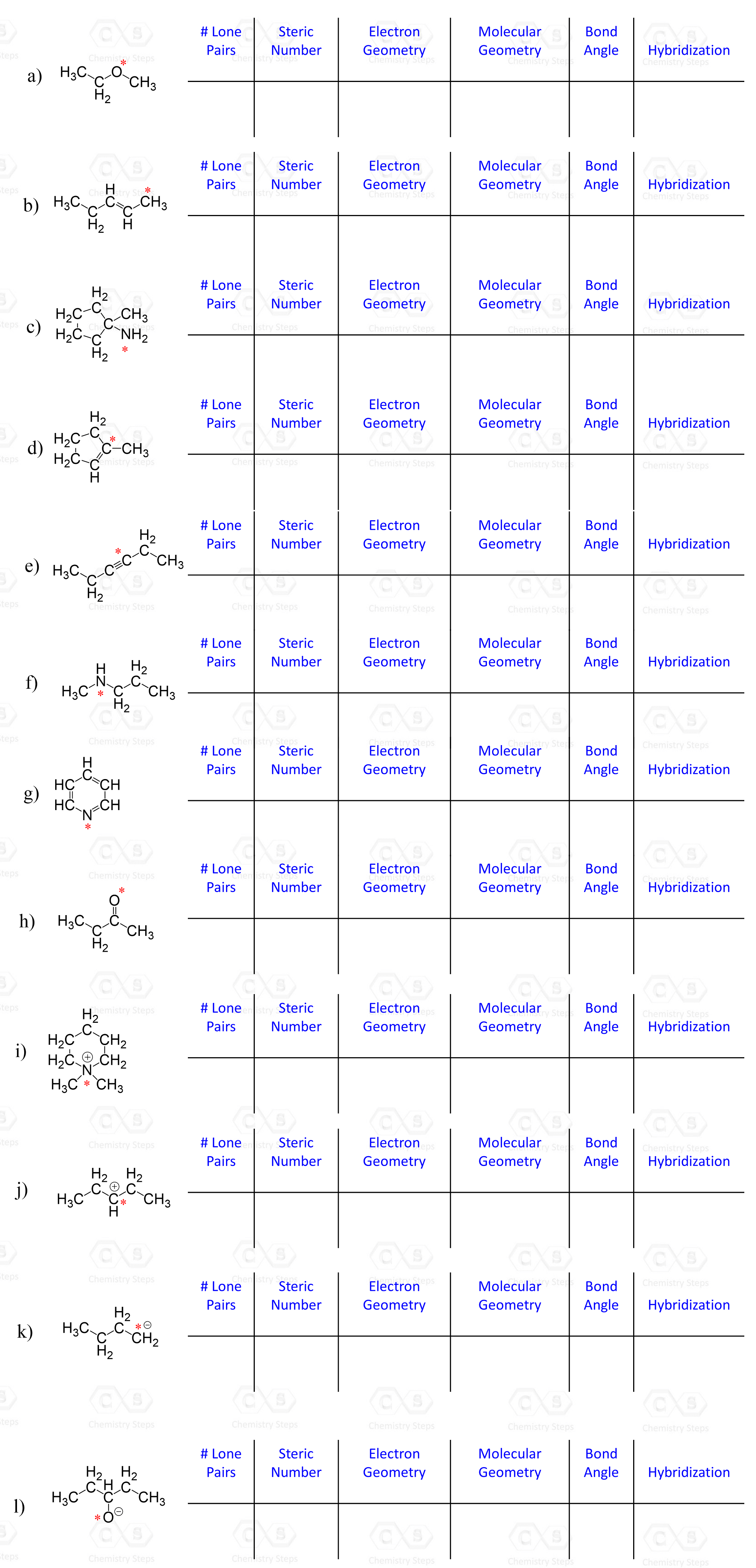
Determine the hybridization state of each carbon and heteroatom (any atom except C and H) in the following compounds.
Hint: Remember to add any missing lone pairs of electrons where necessary.

Check Also
- Lewis Dot Symbols
- The Ionic Bond
- The Covalent Bond
- Sigma and Pi Bonds
- Electronegativity and Bond Polarity
- The Octet Rule
- Formal Charges
- Lewis Structures and the Octet Rule
- Lewis Structures Practice Problems
- Resonance Structures
- The VSEPR Model
- VSEPR Theory Practice Problems
- Hybridization of Atomic Orbitals

for 1n, could SO3’s electron geometry possibly be trigonal planar, with the most stable configuration being 3 S-O double bonds? that way there is no lone pair on Sulfur, and the charges on two oxygens could be eliminated as well. But then SO3’s shape would be trigonal planar and sp3
**trig planar; sp2
Hello,
Yes, it definitely is trigonal planar as there is no lone pair on the sulfur. Please see the updated information. The reason for the mistake was I saw it as SO32- ion which has more electrons. For this, you can check the VSEPR examples here. Thanks for letting me know.