In the previous post, when discussing covalent bonds, we mentioned that the bonding electrons are shared between the atoms. From this statement, and the Lewis structures, which are simplified but a very useful tool in representing molecules, one may understand that the electrons are shared equally between these atoms. In other words, it may seem like the electrons are right in the middle of the two bonded atoms.
This may be a correct interpretation for some molecules where two identical atoms are connected, but it does not explain the experimental data showing that in many molecules the atoms have what’s called partial charges.
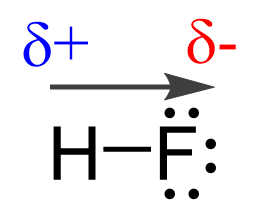
For example, if we place HF in an electrical field, it is shown experimentally that the molecules align such that the H points to the negative, and F to the positive field.
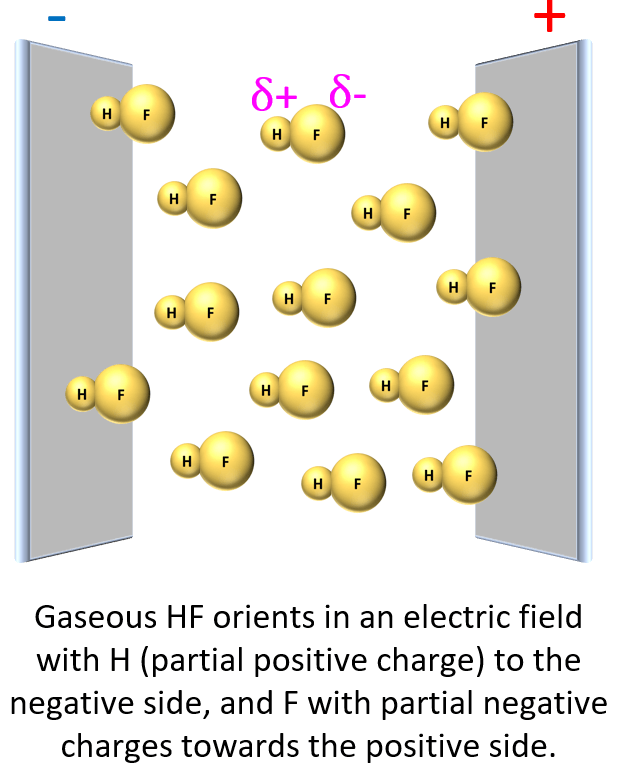
This suggests that the fluorine is more electron-rich than the hydrogen which, in turn, indicates that the electron pair is not equally shared but rather leans towards the F:
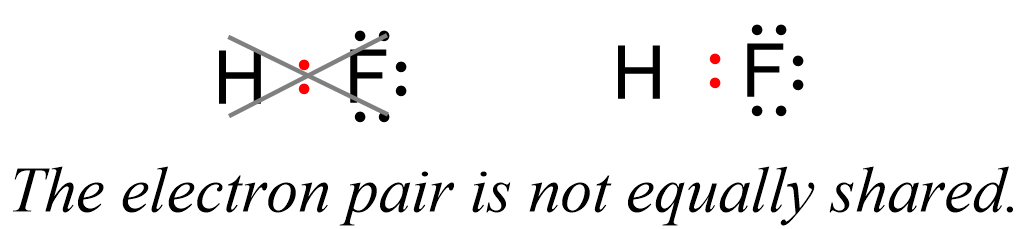
Now, the hydrogen and fluorine are not fully charged, otherwise we’d have an ionic bond. However, we can say that they are “a little bit charged”. This we call partial charges, and they simply show that one atom has a higher electron density that the other. So, in this case, F has a higher electron density, and therefore, it has a partial negative charge (δ-), while hydrogen is partially positively charged (δ+) as it is less electron-dense:
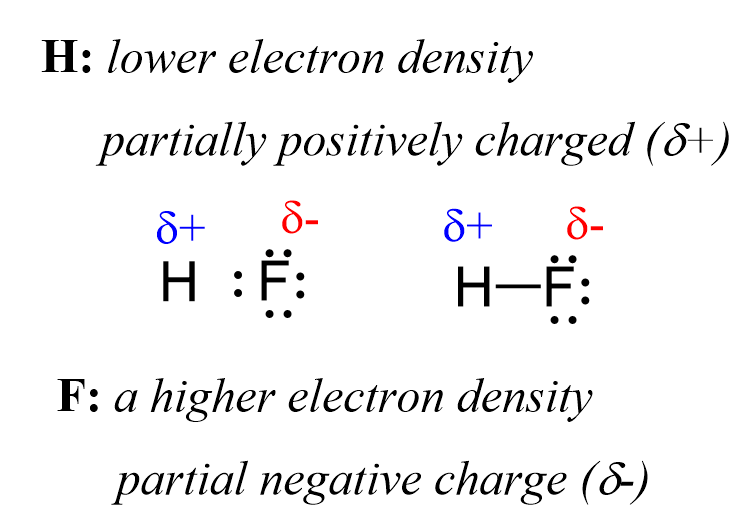
The H-F bond is considered a polar covalent bond because it forms between atoms with greatly different electronegativities. In contrast, when two fluorine atoms are connected, they do not have this unequal electron distribution, and the bond is classified as nonpolar.
Electronegativity
So, why are the electrons shifted to the fluorine? This is explained by electronegativity which is the ability of an atom to attract electrons to itself in a chemical bond.
The higher the electronegative the stronger the atom pulls the electrons. It increases up the column and to the right in the periodical table making fluorine the most electronegative atom. The electronegativity of fluorine is assigned to 4, and the values for the other elements are given relative to that.

Notice that metals have low electronegativity and sometimes we say that they are electropositive. When combined with nonmetals, metals form an ionic bond because the electronegativity of nonmetals is so much larger that they simply take the electron(s) from the metal. The more accurate term, in this case, is electron affinity since we mentioned that electronegativity is the ability of an element to pull the electrons of a chemical bond. In general, electronegativity is related to electron affinity and ionization energy.
Polar and Nonpolar Covalent Bonds
If we visualize the types of bonds we talked about so far, we can place an example of each and realize that the classifications essentially depend on the electronegativity of the atoms.
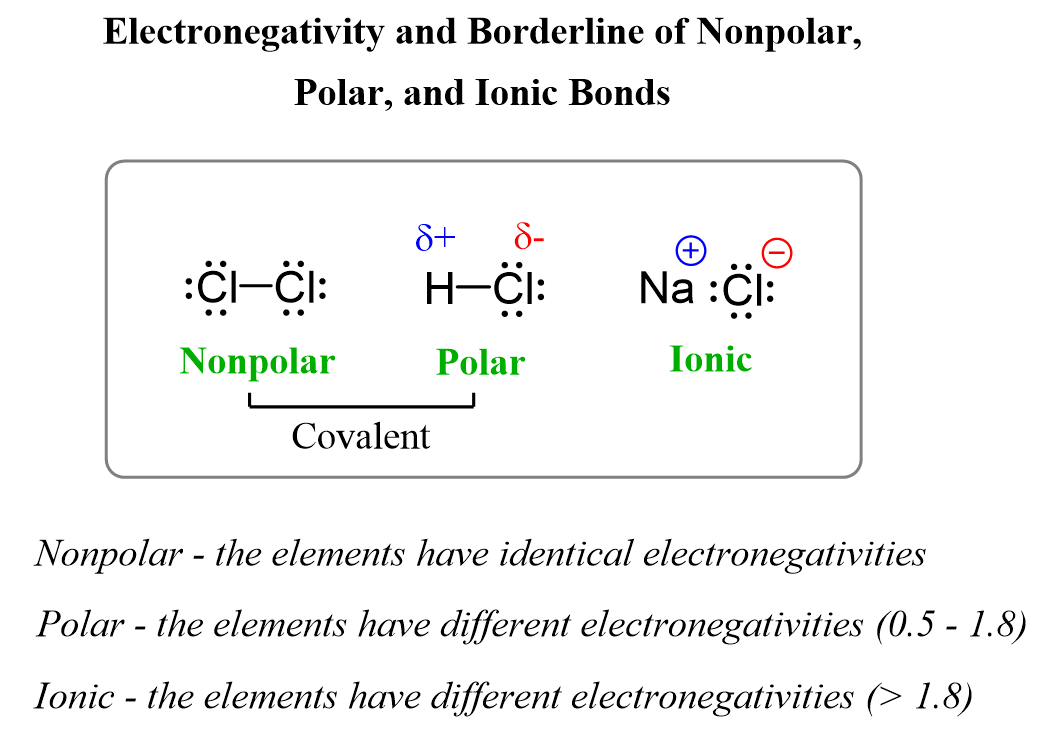
When the electronegativity is equal, we have a nonpolar covalent bond with no charges at all. As the difference increases, we now get to the polar covalent bonds where the atoms are characterized with partial charges. Lastly, when the difference is very large, the more electronegative atom simply takes the electron from the other atom, and two ions are formed which stick together because of electrostatic interactions.
Polar covalent bonds are also characterized by a dipole moment which is a vector quantity calculated by the product of the charge Q and the distance r between the charges:
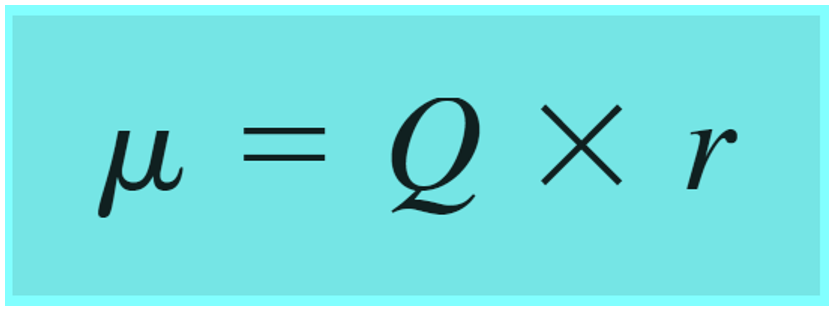
Quantitatively, the vector moment is shown as an arrow on top of the bond pointing towards the more electronegative element.
Is the Bond Ionic, Polar, or Nonpolar?
You may wonder what “great, large” and “little” difference is, and how large the electronegativity difference should actually be to consider a bond polar or ionic. This often causes confusion; however, it is accepted that a bond is polar when the electronegativity difference between two atoms is ≥ 0.5 units. Anything of less difference is considered a nonpolar bond.
It is possible to have a molecule with both polar and nonpolar covalent bonds. One of the most common nonpolar bonds is the C-H bond since it counts to most organic molecules. The C-F bond, on the other hand, is a polar covalent bond. So, in fluoromethane, CFH3, we have three nonpolar and one polar bond:

The direction and magnitude of polarity in a bond are given by the dipole moment which is indicated by an arrow. The arrow starts from the less electronegative element and points toward the more electronegative element as shown above.
Electrostatic Maps
The electron density of a polar covalent bond can also be shown with electrostatic maps. These are simply color-coded clouds where the blue usually corresponds to the most electron-deficient, and the red, to the most electron-rich region.
For example, in fluoromethane, shown above, the fluorine atom has the highest electron density and the carbon atom has less, so this is how the electrostatic map would look like:

As expected, the electron-rich F atom is in the red region since, being more electronegative, it pulls the electron density and thus makes the carbon atom electron deficient.
Check Also
- Lewis Dot Symbols
- The Ionic Bond
- The Covalent Bond
- Sigma and Pi Bonds
- The Octet Rule
- Formal Charges
- Lewis Structures and the Octet Rule
- Lewis Structures Practice Problems
- Resonance Structures
- The VSEPR Model
- VSEPR Theory Practice Problems
- Hybridization of Atomic Orbitals
- sp, sp2, sp3, sp3d, and sp3d2Hybridization Practice Problems
Check this 90-question, Multiple-Choice Quiz on Chemical Bonding:
FreeChemical Bonding and Lewis Structures Quiz

