In the previous two posts, we discussed converting grams to moles and grams to molecules. For the moles, here is what we do: first look up the molar mass in the periodic table, set up the correct conversion factor, and finally do the multiplication. Here is a short summary for converting the mass to moles:
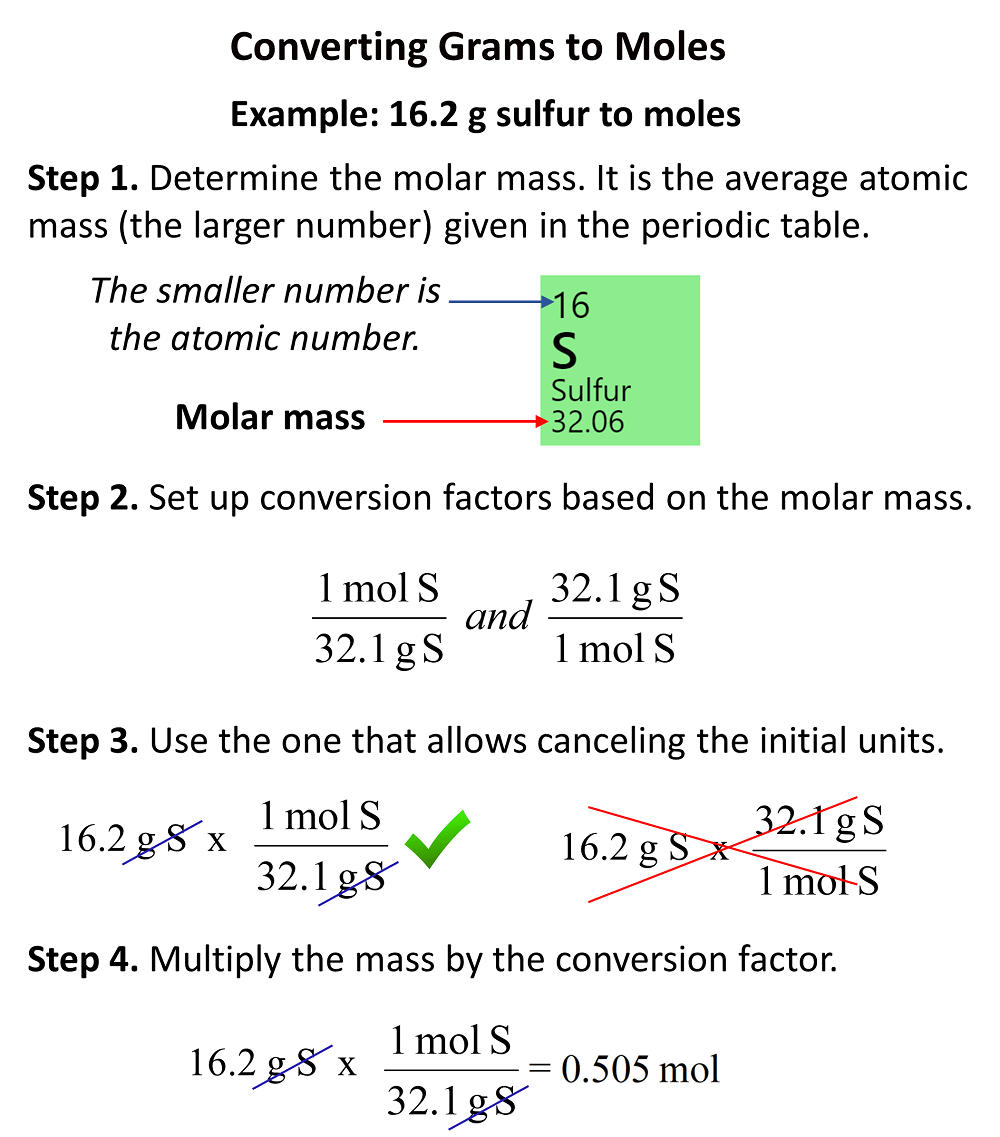
Notice that the exact answer in the calculator is 0.5046728 and we round it off to three significant figures because both initial numbers contain three significant figures.
To calculate the number of molecules or atoms from mass, we need one extra step using the Avogadro’s number (6.02 x 1023). Remember, the Avogadro’s number shows how many particles, which can be atoms, molecules, or ions, there are in one mole of a sample and because it is related to moles, we need to first convert the mass to moles.
So, there are two steps combined in this conversion and the plan is to first convert the mass to moles and then to the number of atoms using NA:
For example, how many atoms of sulfur are there in its 5.20 g sample?
Just like in the summary example, we are first going to determine the number of moles. The molar mass of sulfur is 32.1 g/mol, and therefore, the conversion factors are:
\[\frac{{{\rm{1}}\;{\rm{mol}}\;{\rm{S}}}}{{{\rm{32}}{\rm{.1}}\;{\rm{g}}\,{\rm{S}}}}\;\,and\,\,\frac{{{\rm{32}}{\rm{.1}}\;{\rm{g}}\,{\rm{S}}}}{{{\rm{1}}\;{\rm{mol}}\;{\rm{S}}}}\]
These two simply indicate that 1 mol of S weighs 32.1 g, which is the molar mass of the sulfur which we find in the periodic table.
Now, to calculate the number of moles, we need to choose the correct conversion factor. Remember, the idea is to choose the one that allows canceling the gram units. So, it should be the one where the grams are in the denominator.
\[{\rm{n}}\,{\rm{(S)}}\,{\rm{ = }}\,{\rm{5}}{\rm{.20}}\,{\rm{g}}\,{\rm{ \times }}\,\frac{{{\rm{1}}\;{\rm{mol}}\;{\rm{S}}}}{{{\rm{32}}{\rm{.1}}\;{\rm{g}}\,{\rm{S}}}}\;{\rm{ = }}\,{\rm{0}}{\rm{.162}}\,{\rm{mol}}\]
Once we have the number of moles, we use another conversion factor linking it with the Avogadro’s number.
\[\frac{{{\rm{1}}\;{\rm{mol}}\;{\rm{S}}}}{{{\rm{6}}{\rm{.03}}\;{\rm{ \times }}\,{\rm{1}}{{\rm{0}}^{{\rm{23}}}}\;{\rm{atoms of}}\,{\rm{S}}}}\;{\rm{or}}\,\frac{{{\rm{6}}{\rm{.03}}\;{\rm{ \times }}\,{\rm{1}}{{\rm{0}}^{{\rm{23}}}}\;{\rm{atoms of}}\,{\rm{S}}}}{{{\rm{1}}\;{\rm{mol}}\;{\rm{S}}}}\]
And this time, we need to pick the one that allows canceling the mols which is the second conversion factor:
\[{\rm{0}}{\rm{.162 }}\cancel{{{\rm{mol}}\;{\rm{S}}}}\, \times \;\frac{{{\rm{6}}{\rm{.02}}\;{\rm{ \times }}\,{\rm{1}}{{\rm{0}}^{{\rm{23}}}}\;{\rm{atoms}}\,{\rm{of}}\,{\rm{S}}}}{{{\rm{1}}\;\cancel{{{\rm{mol}}\;{\rm{S}}}}}}\, = \;9.75\,{\rm{ \times }}\,{\rm{1}}{{\rm{0}}^{{\rm{22}}}}\,{\rm{atoms of}}\,{\rm{S}}\]
These conversions can also be combined in one step:
\[{\rm{N}}\,{\rm{(S)}}\,{\rm{ = }}\,{\rm{5}}{\rm{.20}}\,\cancel{{{\rm{g}}\,{\rm{S}}}}\;{\rm{ \times }}\,\frac{{{\rm{1}}\;\cancel{{{\rm{mol}}\;{\rm{S}}}}}}{{{\rm{32}}{\rm{.1}}\;\cancel{{{\rm{g}}\,{\rm{S}}}}}}\,{\rm{ \times }}\;\frac{{{\rm{6}}{\rm{.02}}\;{\rm{ \times }}\,{\rm{1}}{{\rm{0}}^{{\rm{23}}}}\;{\rm{atoms}}\,{\rm{of}}\,{\rm{S}}}}{{{\rm{1}}\;\cancel{{{\rm{mol}}\;{\rm{S}}}}}}\,{\rm{ = }}\;{\rm{9}}{\rm{.75}}\,{\rm{ \times }}\,{\rm{1}}{{\rm{0}}^{{\rm{22}}}}\,{\rm{atoms of}}\,{\rm{S}}\]
Number of Atoms in a Molecule
Sometimes, you may be asked to calculate the number of atoms for a particular element in a molecule. For example, how many atoms of Cl are there in a 54.0 g sample?
As always, first, we determine the molar mass for calculating the number of moles:
M (PCl5) = M (P) + 5 M (Cl) = 31.0 + 5 x 35.5 = 208.5 g/mol
The two conversion factors for calculating the moles would be:
\[\frac{{{\rm{1}}\;{\rm{mol}}\;{\rm{PC}}{{\rm{l}}_{\rm{5}}}}}{{{\rm{208}}{\rm{.5}}\;{\rm{g}}\,{\rm{PC}}{{\rm{l}}_{\rm{5}}}}}\;{\rm{or}}\,\,\frac{{{\rm{208}}{\rm{.5}}\;{\rm{g}}\,{\rm{PC}}{{\rm{l}}_{\rm{5}}}}}{{{\rm{1}}\;{\rm{mol}}\;{\rm{PC}}{{\rm{l}}_{\rm{5}}}}}\]
We are going to use the first conversion factor because it has the units of grams on the denominator which allows us to cancel them with the initial amount given in grams:
\[{\rm{n}}\,{\rm{(PC}}{{\rm{l}}_{\rm{5}}}{\rm{)}}\,{\rm{ = }}\,{\rm{54}}{\rm{.0}}\,\cancel{{{\rm{g}}\,{\rm{PC}}{{\rm{l}}_{\rm{5}}}}}\,{\rm{ \times }}\,\frac{{{\rm{1}}\;{\rm{mol}}\;{\rm{PC}}{{\rm{l}}_{\rm{5}}}}}{{{\rm{208}}{\rm{.5}}\;\cancel{{{\rm{g}}\,{\rm{PC}}{{\rm{l}}_{\rm{5}}}}}}}\;{\rm{ = }}\,{\rm{0}}{\rm{.260}}\,{\rm{mol}}\]
Once we have the number of moles, we can now use it to calculate the number of molecules using another conversion with the Avogadro’s number.
\[\frac{{{\rm{1}}\;{\rm{mol}}\;{\rm{PC}}{{\rm{l}}_{\rm{5}}}}}{{{\rm{6}}{\rm{.02}}\;{\rm{ \times }}\,{\rm{1}}{{\rm{0}}^{{\rm{23}}}}\;{\rm{molecules}}\;{\rm{PC}}{{\rm{l}}_{\rm{5}}}}}\;{\rm{or}}\,\frac{{{\rm{6}}{\rm{.02}}\;{\rm{ \times }}\,{\rm{1}}{{\rm{0}}^{{\rm{23}}}}\;{\rm{molecules}}\,{\rm{PC}}{{\rm{l}}_{\rm{5}}}}}{{{\rm{1}}\;{\rm{mol}}\;{\rm{PC}}{{\rm{l}}_{\rm{5}}}}}\]
And this time, we need to pick the one that allows canceling the mols which is the second conversion factor:
\[{\rm{0}}{\rm{.26 }}\cancel{{{\rm{mol}}\;{\rm{PC}}{{\rm{l}}_{\rm{5}}}}}\, \times \;\frac{{{\rm{6}}{\rm{.02}}\;{\rm{ \times }}\,{\rm{1}}{{\rm{0}}^{{\rm{23}}}}\;{\rm{molecules}}\,{\rm{PC}}{{\rm{l}}_{\rm{5}}}}}{{{\rm{1}}\;\cancel{{{\rm{mol}}\;{\rm{PC}}{{\rm{l}}_{\rm{5}}}}}}}\, = \;1.57\,{\rm{ \times }}\,{\rm{1}}{{\rm{0}}^{{\rm{23}}}}\,{\rm{molecules PC}}{{\rm{l}}_{\rm{5}}}\]
In the last step, we are going to calculate the number of Cl atoms in 1.57 x 1023 molecules of PCl5. For this, we need to understand the formula of PCl5. The subscript 5 indicates that there are 5 chlorine atoms in one molecule of PCl5. Therefore, to determine the number of Cl atoms in 1.57 x 1023 molecules of PCl5, we multiply it by 5:
\[{\rm{N}}\,{\rm{(Cl)}}\,{\rm{ = }}\,{\rm{1}}{\rm{.57}}\,{\rm{ \times }}\,{\rm{1}}{{\rm{0}}^{{\rm{23}}}}\,\cancel{{{\rm{molecules}}\;{\rm{of}}\,{\rm{PC}}{{\rm{l}}_{\rm{5}}}}}{\rm{ \times }}\,\frac{{{\rm{5}}\,{\rm{atoms}}\,{\rm{of Cl}}}}{{{\rm{1}}\;\cancel{{{\rm{molecule}}\;{\rm{of}}\;{\rm{PC}}{{\rm{l}}_{\rm{5}}}}}}}\;{\rm{ = }}\;{\rm{7}}{\rm{.85}}\,{\rm{ \times }}\,{\rm{1}}{{\rm{0}}^{{\rm{23}}}}\,{\rm{Cl}}\,{\rm{atoms}}\]
And here is how we can combine the conversions into a one-step process:
\[{\rm{N}}\,{\rm{(PC}}{{\rm{l}}_{\rm{5}}}{\rm{)}}\,{\rm{ = }}\,{\rm{54}}{\rm{.0}}\,\cancel{{{\rm{g}}\,{\rm{PC}}{{\rm{l}}_{\rm{5}}}}}\,{\rm{ \times }}\,\frac{{{\rm{1}}\;\cancel{{{\rm{mol}}\;{\rm{PC}}{{\rm{l}}_{\rm{5}}}}}}}{{{\rm{208}}{\rm{.5}}\;\cancel{{{\rm{g}}\,{\rm{PC}}{{\rm{l}}_{\rm{5}}}}}}}\; \times \;\frac{{{\rm{6}}{\rm{.02}}\;{\rm{ \times }}\,{\rm{1}}{{\rm{0}}^{{\rm{23}}}}\;\cancel{{{\rm{molecules}}\,{\rm{PC}}{{\rm{l}}_{\rm{5}}}}}}}{{{\rm{1}}\;\cancel{{{\rm{mol}}\;{\rm{PC}}{{\rm{l}}_{\rm{5}}}}}}}\,{\rm{ \times }}\,\frac{{{\rm{5}}\,{\rm{atoms}}\,{\rm{of Cl}}}}{{{\rm{1}}\;\cancel{{{\rm{molecule}}\;{\rm{of}}\;{\rm{PC}}{{\rm{l}}_{\rm{5}}}}}}}\;{\rm{ = }}\;{\rm{7}}{\rm{.85}}\,{\rm{ \times }}\,{\rm{1}}{{\rm{0}}^{{\rm{23}}}}\,{\rm{Cl}}\,{\rm{atoms}}\,\]
Another example: How many atoms of oxygen are there in a 35.0 g sample of glucose, C6H12O6?
The molar mass of glucose is:
M (C6H12O6) = 6 x 12 + 12 x 1.0 + 6 x 16 = 180 g/mol
Therefore, the two conversion factors for going mass → moles, and moles → molecules are:
\[\frac{{{\rm{1}}\,{\rm{mol}}\,{{\rm{C}}_{\rm{6}}}{{\rm{H}}_{{\rm{12}}}}{{\rm{O}}_{\rm{6}}}}}{{{\rm{180}}{\rm{.}}\,{\rm{g}}}}\;,\,\,\frac{{{\rm{6}}{\rm{.022}}\;{\rm{ \times }}\,{\rm{1}}{{\rm{0}}^{{\rm{23}}}}\;{{\rm{C}}_{\rm{6}}}{{\rm{H}}_{{\rm{12}}}}{{\rm{O}}_{\rm{6}}}\,{\rm{molecules}}}}{{{\rm{1}}\,{\rm{mol}}\,{{\rm{C}}_{\rm{6}}}{{\rm{H}}_{{\rm{12}}}}{{\rm{O}}_{\rm{6}}}}}\]
So, we can use these factors to convert the mass to the number of molecules:
\[{\rm{N}}\;{\rm{(}}{{\rm{C}}_{\rm{6}}}{{\rm{H}}_{{\rm{12}}}}{{\rm{O}}_{\rm{6}}}{\rm{)}}\,{\rm{ = }}\;{\rm{35}}{\rm{.0}}\cancel{{\rm{g}}}\;{\rm{ \times }}\,\frac{{{\rm{1}}\cancel{{{\rm{mol}}\,{{\rm{C}}_{\rm{6}}}{{\rm{H}}_{{\rm{12}}}}{{\rm{O}}_{\rm{6}}}}}}}{{{\rm{180}}{\rm{.}}\cancel{{\rm{g}}}}}\;{\rm{ \times }}\,\frac{{{\rm{6}}{\rm{.022}}\;{\rm{ \times }}\,{\rm{1}}{{\rm{0}}^{{\rm{23}}}}\;{{\rm{C}}_{\rm{6}}}{{\rm{H}}_{{\rm{12}}}}{{\rm{O}}_{\rm{6}}}\,{\rm{molecules}}}}{{{\rm{1}}\,\cancel{{{\rm{mol}}\,{{\rm{C}}_{\rm{6}}}{{\rm{H}}_{{\rm{12}}}}{{\rm{O}}_{\rm{6}}}}}}}\; = \;1.17\;{\rm{ \times }}\,{\rm{1}}{{\rm{0}}^{{\rm{23}}}}\;\;\;\]
To find the number of oxygen atoms, we multiply this number by 6 because there are 6 oxygen atoms in molecules of glucose. Combining all the steps together, we can write the following conversion sequence:
\[{\rm{N}}\;{\rm{(O)}}\,{\rm{ = }}\;{\rm{35}}{\rm{.0}}\cancel{{{\rm{g}}\;{{\rm{C}}_{\rm{6}}}{{\rm{H}}_{{\rm{12}}}}{{\rm{O}}_{\rm{6}}}}}\;{\rm{ \times }}\,\frac{{{\rm{1}}\cancel{{{\rm{mol}}\,{{\rm{C}}_{\rm{6}}}{{\rm{H}}_{{\rm{12}}}}{{\rm{O}}_{\rm{6}}}}}}}{{{\rm{180}}{\rm{.}}\cancel{{\rm{g}}}}}\;{\rm{ \times }}\,\frac{{{\rm{6}}{\rm{.022}}\;{\rm{ \times }}\,{\rm{1}}{{\rm{0}}^{{\rm{23}}}}\;\cancel{{{{\rm{C}}_{\rm{6}}}{{\rm{H}}_{{\rm{12}}}}{{\rm{O}}_{\rm{6}}}\,{\rm{molecules}}}}}}{{{\rm{1}}\,\cancel{{{\rm{mol}}\,{{\rm{C}}_{\rm{6}}}{{\rm{H}}_{{\rm{12}}}}{{\rm{O}}_{\rm{6}}}}}}}\; \times \frac{{{\rm{6}}\,{\rm{atoms}}\,{\rm{of O}}}}{{{\rm{1}}\,\cancel{{{{\rm{C}}_{\rm{6}}}{{\rm{H}}_{{\rm{12}}}}{{\rm{O}}_{\rm{6}}}\,{\rm{molecule}}}}}}\, = \;7.02\;{\rm{ \times }}\,{\rm{1}}{{\rm{0}}^{{\rm{23}}}}\,{\rm{atoms}}\,{\rm{of O}}\;\;\;\]
Converting the Number of Atoms to Grams
Another type of question relating the mass and the number of atoms is to determine the mass of a sample given the number of atoms. This is the reverse calculation, and we are going to use the reciprocal of the conversion factors that we used in the previous calculations.
For example, let’s say we were asked to determine the mass of an iron sample that contains 7.58 x 1024 atoms. These are the conversion factors that we are going to need:

The first one allows converting the number of atoms to moles, and the second is converting the moles to mass.
\[{\rm{7}}{\rm{.58 x 1}}{{\rm{0}}^{{\rm{24}}}}\,\cancel{{{\rm{Fe}}\,{\rm{atoms}}}}\,{\rm{ \times }}\,\frac{{{\rm{1}}\;\cancel{{{\rm{mol}}\;{\rm{Fe}}}}}}{{{\rm{6}}{\rm{.03}}\;{\rm{ \times }}\,{\rm{1}}{{\rm{0}}^{{\rm{23}}}}\;\cancel{{{\rm{Fe}}\;{\rm{atoms}}}}}}\,{\rm{ \times }}\,\frac{{{\rm{55}}{\rm{.8}}\;{\rm{g}}\,{\rm{Fe}}}}{{{\rm{1}}\;\cancel{{{\rm{mol}}\;{\rm{Fe}}}}}}\;{\rm{ = }}\,{\rm{703}}\,{\rm{g}}\;\]
Practice
Calculating Moles from Mass
Determine the number of moles in 59.7 grams of Al.
Determine the number of moles in 2.41 grams of FeO.
Calculate the number of moles in 0.647 grams of Al2O3.
Determine the number of moles in 3.56 grams of Mg(OH)2.
Determine the number of moles in 0.385 grams of N2O3.
Determine the number of moles in 165 grams of CaSO4.
Calculate the molar mass of N2O4 and determine how many moles of it are in a 23.9 g sample.
Calculate the number of moles in 165 grams of C3H6O.
Determine the number of moles in 452 grams of Co(NO3)3.
Calculating Mass from Moles
Calculate the mass in grams of 0.598 moles of Fe.
Calculate the mass in grams of 0.168 moles of NO.
Calculate the mass in grams of 0.987 moles of (NH4)2S.
Calculate the mass in grams of 6.81 moles of Al2(SO4)3.
Calculate the mass in grams of 2.64 moles of methanol, CH3OH.
Calculate the mass in grams of 9.42 moles of NiCl2·6H2O.
Calculating the Number of Molecules from the Moles
How many molecules are there in a 0.487 mol sample of PCl5?
How many molecules (formula units) are there in a 5.84 mol sample of Na2SO3.
How many molecules of sucrose, C12H22O11 are there in a 0.684 mol sample?
Calculate the number of molecules in a 3.25-mol sample of propane, C3H8.
How many moles is 5.80 x 1025 molecules of POCl3?
Calculating the Number of Molecules from the Mass
How many molecules are there in a 5.12-g sample of K2O?
How many molecules of glucose, C6H12O6 are there in a 35.0 g sample?
Calculate the number of molecules of butane, C4H10, in its 2.40-gram sample.
How many Ethylene, C2H4 molecules are present in a 46.2 g sample? The molar mass of C2H4 is 28.1 g/mol.
Calculating the Number of Atoms
Calculate the number of atoms in a 2.56-g sample of Ca.
How many carbon atoms are there in a 0.590 mol sample of CCl4.
How many carbon atoms are there in a 0.964 mol sample of C2H6.
Which sample contains a more Cl atoms: a) 1.25 moles of CH2Cl2 b) 2.15 moles of CH3Cl
The molecular formula of morphine is C17H19NO3. How many carbon atoms are in a 34.7-gram sample of morphine?
Isopropyl alcohol, also known as isopropanol, has found a widespread application in the preparation of pharmaceutical products. Answer the following questions considering that the molecular formula of isopropanol is C3H8O.
a) How many moles of C3H8O are contained in a 12.0 g sample of the alcohol?
b) How many molecules of C3H8O are contained in a 12.0 g sample of the alcohol?
c) How many atoms of oxygen are contained in a 12.0 g sample of the isopropyl alcohol (C3H8O)?
d) How many atoms of carbon are contained in a 12.0 g sample of the isopropyl alcohol (C3H8O)?
Check Also
- Subatomic particles and Isotopes
- Naming Monatomic and Polyatomic Ions
- Naming Ionic Compounds
- Naming Covalent Compounds
- Naming Acids and Bases
- Atomic and Molecular Masses
- The Mole and Molar Mass
- Percent Composition and Empirical Formula
- Stoichiometry of Chemical Reactions
- Limiting Reactant
- Limiting Reactant Practice Problems
- Reaction/Percent Yield
- Stoichiometry Practice Problems
