This is a comprehensive, end-of-chapter set of practice problems on stoichiometry that covers balancing chemical equations, mole-ratio calculations, limiting reactants, and percent yield concepts.
The links to the corresponding topics are given below.
Practice
Balance the following chemical equations:
a) HCl + O2 → H2O + Cl2
b) Al(NO3)3 + NaOH → Al(OH)3 + NaNO3
c) H2 + N2 → NH3
d) PCl5 + H2O → H3PO4 + HCl
e) Fe + H2SO4 → Fe2(SO4)3 + H2
f) CaCl2 + HNO3 → Ca(NO3)2 + HCl
g) KO2 + H2O → KOH + O2 + H2O2
h) Al + H2O → Al2O3 + H2
i) Fe + Br2 → FeBr3
j) Cu + HNO3 → Cu(NO3)2 + NO2 + H2O
k) Al(OH)3 → Al2O3 + H2O
l) NH3 + O2 → NO + H2O
m) Ca(AlO2)2 + HCl → AlCl3 + CaCl2 + H2O
n) C5H12 + O2 → CO2 + H2O
o) P4O10 + H2O → H3PO4
p) Na2CrO4 + Pb(NO3)2 → PbCrO4 + NaNO3
q) MgCl2 + AgNO3 → AgCl + Mg(NO3)2
r) KClO3 → KClO4 + KCl
s) Ca(OH)2 + H3PO4 → Ca3(PO4)2 + H2O
Consider the balanced equation:
C5H12 + 8 O2 → 5CO2 + 6H2O
Complete the table showing the appropriate number of moles of reactants and products.
| mol C5H12 | mol O2 | mol CO2 | mol H2O |
| 2 | |||
| 2.5 | |||
| 3 | |||
| 5.4 |
How many grams of CO2 and H2O are produced from the combustion of 220. g of propane (C3H8)?
C3H8(g) + 5O2(g) → 3CO2(g) + 4H2O(g)
How many grams of CaCl2 can be produced from 65.0 g of Ca(OH)2 according to the following reaction,
Ca(OH)2 + 2HCl → CaCl2 + 2H2O
How many moles of oxygen are formed when 75.0 g of Cu(NO3)2 decomposes according to the following reaction?
2Cu(NO3)2 → 2CuO + 4NO2 + O2
How many grams of MnCl2 can be prepared from 52.1 grams of MnO2?
MnO2 + 4HCl → MnCl2 + Cl2 + 2H2O
Determine the mass of oxygen that is formed when an 18.3-g sample of potassium chlorate is decomposed according to the following equation:
2KClO3(s) → 2KCl(s) + 3O2(g).
How many grams of H2O will be formed when 48.0 grams H2 are mixed with excess hydrogen gas?
2H2 + O2 → 2H2O
Consider the chlorination reaction of methane (CH4):
CH4(g) + 4Cl2(g) → CCl4(g) + 4HCl(g)
How many moles of CH4 were used in the reaction if 51.9 g of CCl4 were obtained?
How many grams of Ba(NO3)2 can be produced by reacting 16.5 g of HNO3 with an excess of Ba(OH)2?
Ethanol can be obtained by fermentation – a complex chemical process breaking down glucose to ethanol and carbon dioxide.
C6H12O6 → 2C2H5OH + 2CO2
glucose ethanol
How many mL of ethanol (d =0.789 g/mL) can be obtained by this process starting with 286 g of glucose?
36.0 g of butane (C4H10) was burned in an excess of oxygen and the resulting carbon dioxide (CO2) was collected in a sealed vessel.
2C4H10 + 13O2 → 8CO2 + 10H2O
How many grams of LiOH will be necessary to consume all the CO2 from the first reaction?
2LiOH + CO2 → Li2CO3 + H2O
Limiting Reactant
13. Which statement about limiting reactant is correct?
a) The limiting reactant is the one in a smaller quantity.
b) The limiting reactant is the one in greater quantity.
c) The limiting reactant is the one producing less product.
d) The limiting reactant is the one producing more product.
Find the limiting reactant for each initial amount of reactants.
4NH3 + 5O2 → 4NO + 6H2O
a) 2 mol of NH3 and 2 mol of O2
b) 2 mol of NH3 and 3 mol of O2
c) 3 mol of NH3 and 3 mol of O2
d) 3 mol of NH3 and 2 mol of O2
Note: This is not a multiple-choice question. Each row represents a separate question where you need to determine the limiting reactant.
How many g of hydrogen are left over in producing ammonia when 14.0 g of nitrogen is reacted with 8.0 g of hydrogen?
N2(g) + 3 H2(g) → 2 NH3(g)
How many grams of PCl3 will be produced if 130.5 g Cl2 is reacted with 56.4 g P4 according to the following equation?
6Cl2(g) + P4(s) → 4PCl3(l)
How many grams of sulfur can be obtained if 12.6 g H2S is reacted with 14.6 g SO2 according to the following equation?
2H2S(g) + SO2(g) → 3S(s) + 2H2O(g)
The following equation represents the combustion of octane, C8H18, a component of gasoline:
2C8H18(g) + 25O2(g) → 16CO2(g) + 18H2O(g)
Will 356 g of oxygen be enough for the complete combustion of 954 g of octane?
When 140.0 g of AgNO3 was added to an aqueous solution of NaCl, 86.0 g of AgCl was collected as a white precipitate. Which salt was the limiting reactant in this reaction? How many grams of NaCl were present in the solution when AgNO3 was added?
AgNO3(aq) + NaCl(aq) → AgCl(s) + NaNO3(aq)
Consider the reaction between MnO2 and HCl:
MnO2 + 4HCl → MnCl2 + Cl2 + 2H2O
What is the theoretical yield of MnCl2 in grams when 165 g of MnO2 is added to a solution containing 94.2 g of HCl?
Percent Yield
21. In a chemistry experiment, a student obtained 5.68 g of a product. What is the percent yield of the product if the theoretical yield was 7.12 g?
When 38.45 g CCl4 is reacted with an excess of HF, 21.3 g CCl2F2 is obtained. Calculate the theoretical and percent yields of this reaction.
CCl4 + 2HF → CCl2F2 + 2HCl
Iron(III) oxide reacts with carbon monoxide according to the equation:
Fe2O3(s) + 3CO(g) → 2Fe(s) + 3CO2(g)
What is the percent yield of this reaction if 623 g of iron oxide produces 341 g of iron?
Determine the percent yield of the reaction if 77.0 g of CO2 are formed from burning 2.00 moles of C5H12 in 4.00 moles of O2.
C5H12 + 8 O2 → 5CO2 + 6H2O
The percent yield for the following reaction was determined to be 84%:
N2(g) + 2H2(g) → N2H4(l)
How many grams of hydrazine (N2H4) can be produced when 38.36 g of nitrogen reacts with 6.68 g of hydrogen?
Silver metal can be prepared by reducing its nitrate, AgNO3 with copper according to the following equation:
Cu(s) + 2AgNO3(aq) → Cu(NO3)2(aq) + 2Ag(s)
What is the percent yield of the reaction if 71.5 grams of Ag was obtained from 132.5 grams of AgNO3 ?
Industrially, nitric acid is produced from ammonia by the Ostwald process in a series of reactions:
4NH3(g) + 5O2(g) → 4NO(g) + 6H2O(l)
2NO(g) + O2(g) → 2NO2(g)
2NO2(g) + H2O(l) → HNO3(aq) + HNO2(aq)
Considering that each reaction has an 85% percent yield, how many grams of NH3 must be used to produce 25.0 kg of HNO3 by the above procedure?
Aspirin (acetylsalicylic acid) is widely used to treat pain, fever, and inflammation. It is produced from the reaction of salicylic acid with acetic anhydride. The chemical equation for aspirin synthesis is shown below:
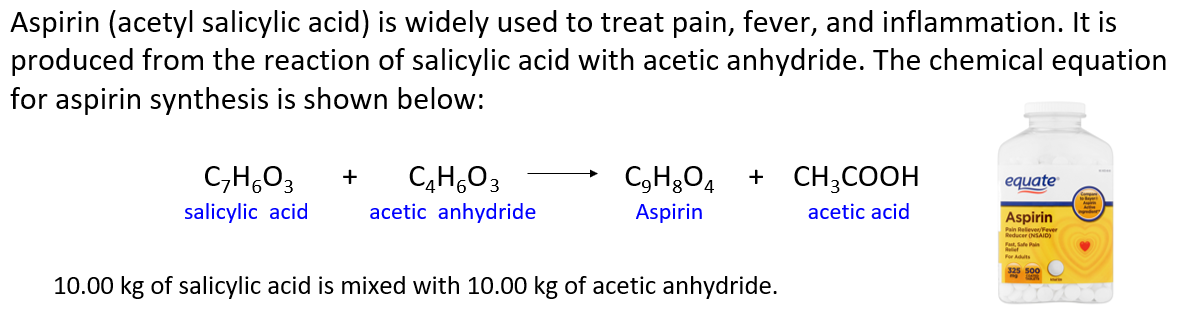
In one container, 10.00 kg of salicylic acid is mixed with 10.00 kg of acetic anhydride.
a) Which reactant is limiting? Which is in excess?
b) What mass of excess reactant is left over?
c) What mass of aspirin is formed assuming 100% yield (Theoretical yield)?
d) What mass of aspirin is formed if the reaction yield is 70.0% ?
e) If the actual yield of aspirin is 11.2 kg, what is the percent yield?
f) How many kg of salicylic acid is needed to produce 20.0 kg of aspirin if the reaction yield is 85.0% ?


You forgot the subscript 3 for O in the molecular formula for acetic anhydride and the reaction is not balanced as written.
For part F) it’s 18.1 kg and not1.81 kg as written in the final line of the solution.
Thanks for letting me know! Fixed.
You’re welcome!