Naming Monoatomic Cations and Anions
For naming purposes, ions can be divided into two types: monatomic (monoatomic) and polyatomic ions. Monatomic ions are formed when an element gains or loses an electron(s).
Metals tend to lose electron(s) and become cations (positively charged ions).
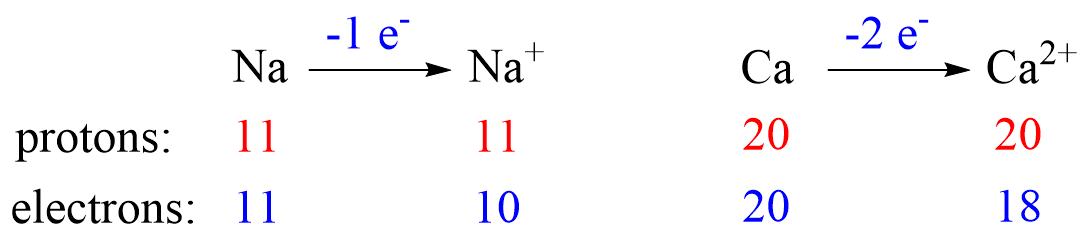
Nonmetals tend to gain an electron(s) and become anions (negatively charged ions).
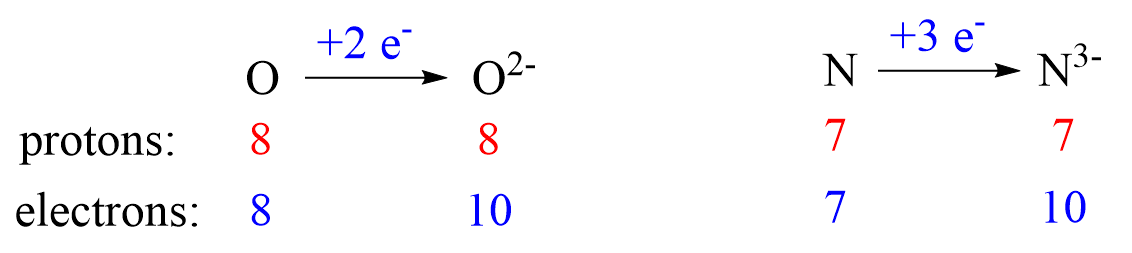
With the exception of ammonia (NH4+), all the metals are cations. Most cations simply inherit the name of the metal they are derived from:
Li+ = lithium ion, Al3+ = aluminum ion, Ca2+ = calcium ion
The ionic charge depends on the nature of the element. Remember the following ions in groups 1A, 2A, and 7A as their charges are predictable and go based on their group:
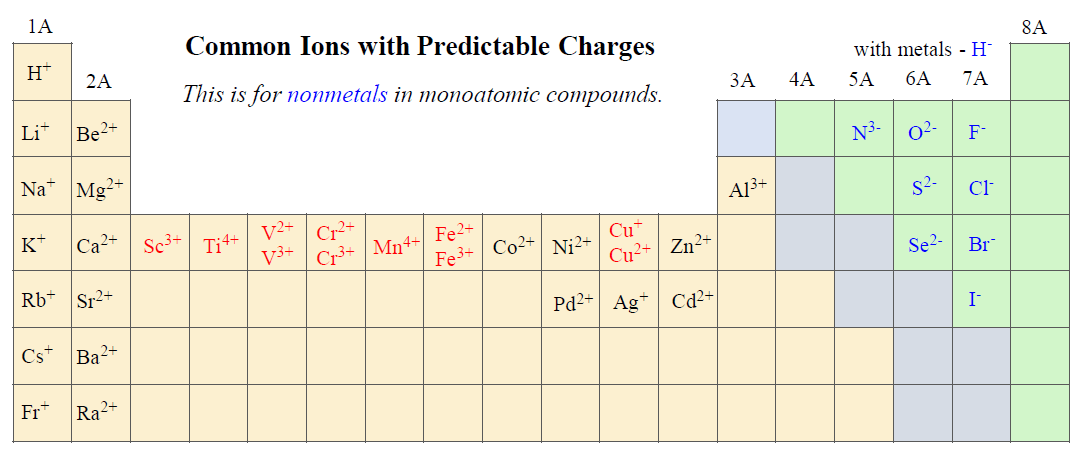
To name a monatomic anion, we change the ending to “ide”. For example,
Nitrogen (N2) – Nitride (N3-)
Oxygen (O2) – Oxide (O2-)
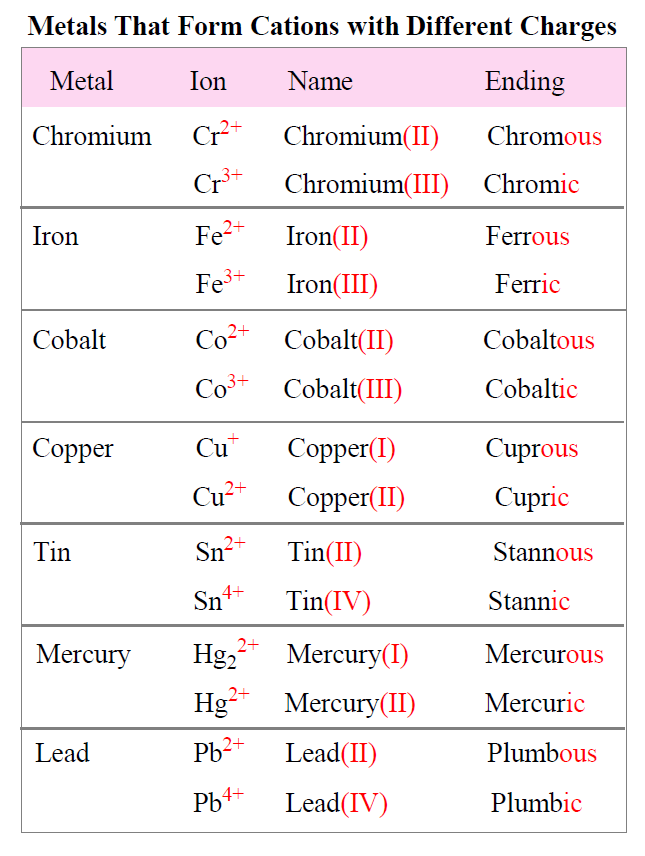
Cations with Different Charges
Unlike group 1A and 2A metals, some transition metals can form cations with different charges. Therefore, their positive charge is indicated either by a Roman numeral before the name of the metal or by their suffix.
For example, iron can for Fe2+ and Fe3+ ions. For the Roman numeral, they would be named iron(II) and iron(III) respectively. The number provides information about the charge.
The suffix for the cation with a higher charge is “ic” and for the lower charge is “ous”, so they will be ferric for Fe3+, and ferrous for Fe2+.
The names and formulas of metals forming different cations is summarized in the table below:
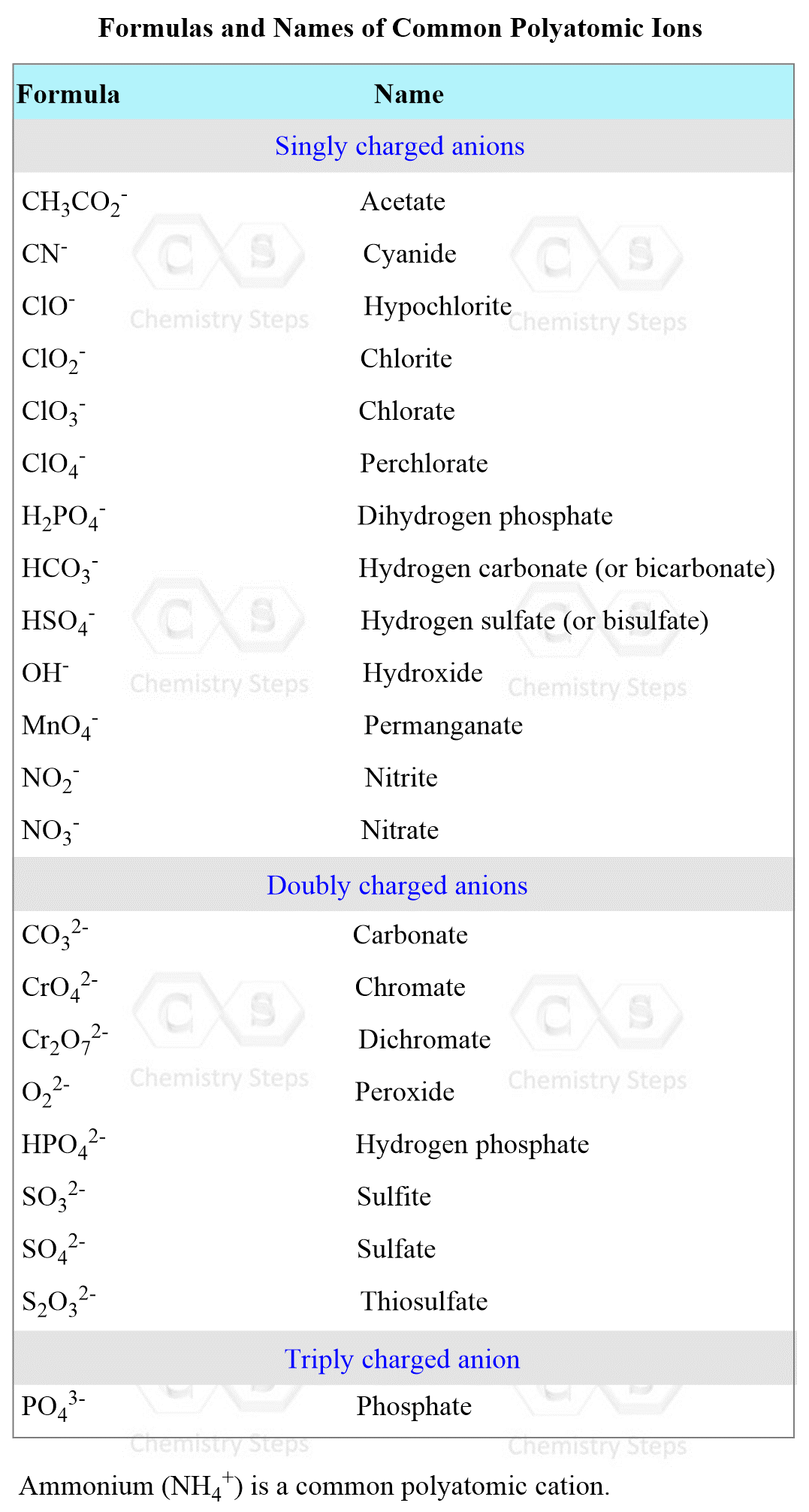
Naming Polyatomic Ions
Polyatomic ions are also called oxyanions or oxoanions because they contain at least one oxygen atom. For example,
SO42- – sulfate, NO2– – nitrite, ClO4– – perchlorate
Note that the ending (suffix) changes and sometimes there is a prefix in addition to the suffix. This depends on the number of oxygen atoms in the ions.
- If there are only two ions in the series, the one with more oxygen atoms takes the ending -ate, and the one with fewer takes the ending -ite. For example, nitrogen makes an oxyanion with two and three oxygens. The names, therefore, are:
NO3– – nitrate and NO2– – nitrite
Sulfur is another example, forming two oxyanions:
SO42- – sulfate, SO32- – sulfite
- If the atom forms more than two oxoanions, the prefix hypo– (meaning “less than”) is used for the ion with the fewest oxygens, and per– ( “more than”) is used for the ion with the most oxygens. The other two anions are given prefixes “ate” and “ite” as we saw earlier.
For example,
ClO– – Hypochlorite ion (fewest oxygena)
ClO2– – Chlorite ion
ClO3– – Chlorate ion
ClO4– – Perchlorate iron (most oxygens)
Some oxyanions form a derivative by a presence of a hydrogen ion which is named by using the prefix “bi”, For example,
CO32- Carbonate ion, HCO3– Bicarbonate
SO42- – sulfate ion, HSO4– – bisulfate ion
There are a few ions, hydroxide (OH–) and cyanide (CN–), and peroxide (O22–) have the ending –ide.
These rules may seem overwhelming, and they probably are, but at the end of the day, you are just going to memorize the names of the ions and not have to go back to the rules.
Below is the list of the formulas and names of the polyatomic ions:
More practice examples on nomenclature and formulas can be found in this multiple-choice quiz:
Check Also

