In the previous post, we talked about ionization energy which is the amount of energy required to remove an electron from the isolated neutral atom in the gaseous state.
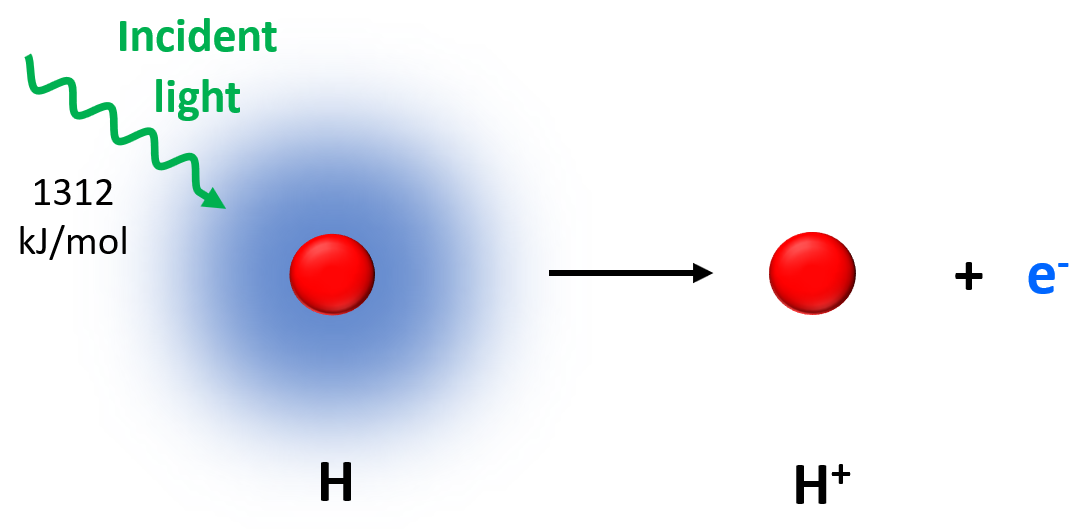
During the ionization, a neutral atom was becoming a cation and if we kept shining light with sufficient energy, consecutive removal of electrons produced cations with greater charges.
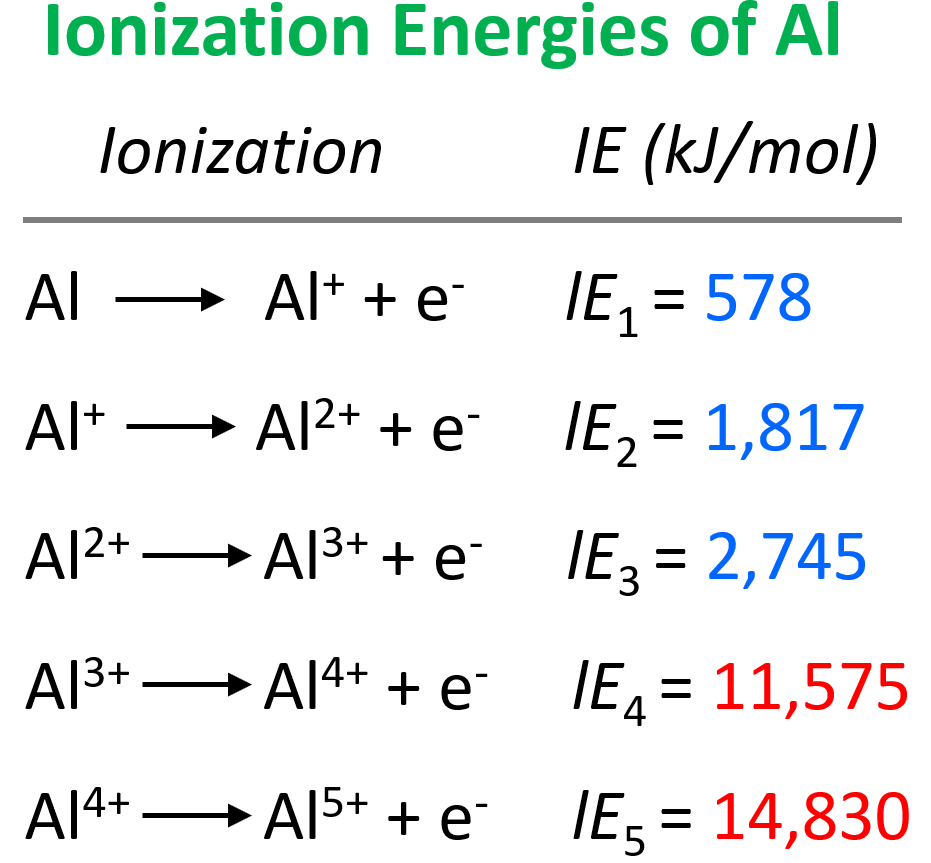
Notice that all the ionization energies are positive and therefore, removing an electron is almost always an endothermic process. In other words, energy is needed to remove an electron from an atom or especially from a cation.
It turns out, on the other hand, that for a lot of atoms, energy is released when an electron is added to one of its orbitals. For example, 349 kJ of energy is released one mole of chlorine atoms become chloride ions:
![]()
This is because most atoms “want” to add an electron because of their nuclear charge. This is the electron affinity which is the energy change that occurs when an electron is added to a gaseous atom.
The electron affinity is somewhat the opposite of ionization energy; however, it is not the reverse process. Remember, in ionization, we remove an electron from an atom forming a cation, while here, the electron is added to the atom forming an anion.
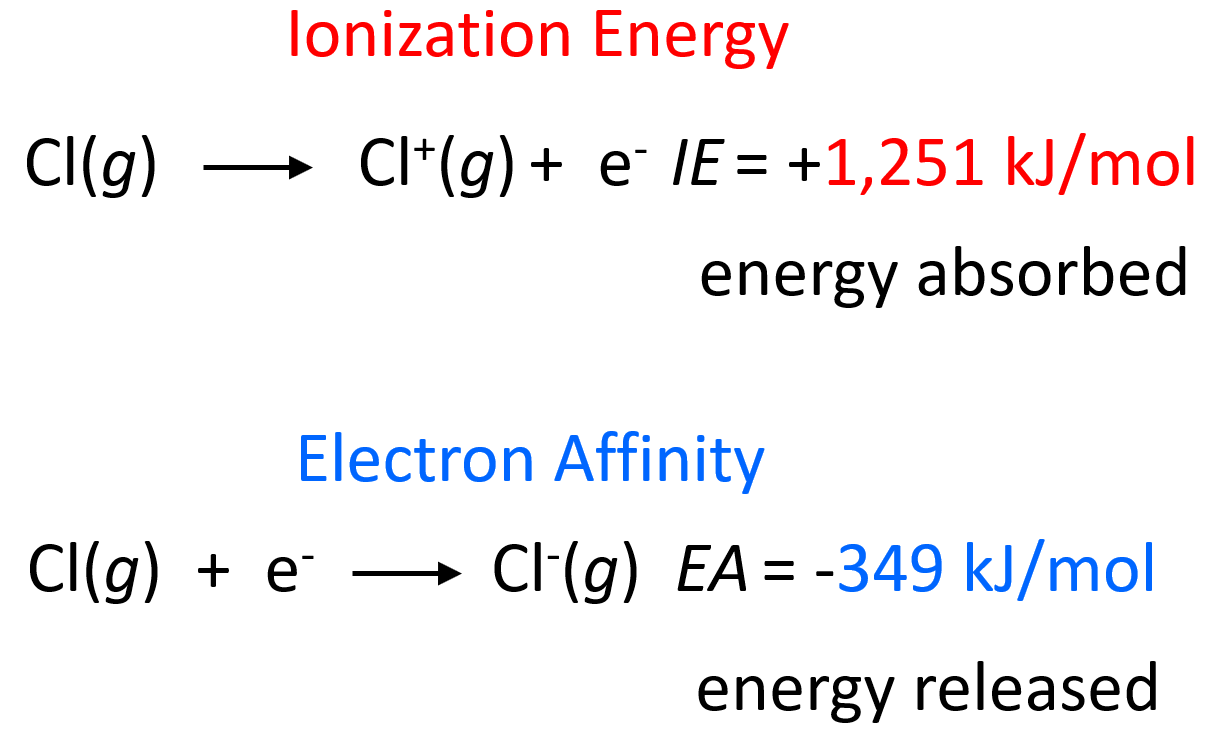
If they were reverse processes, then the absorbed and released energies would have had the same values but opposite signs.
Periodic Trends of Electron Affinity
There are many exceptions and deviations in the behavior of atoms toward adding an electron(s). However, let’s try to understand what properties of atoms will make them good candidates for accepting electrons. Overall, we are looking at two factors: the nuclear charge and the size of the atom. We expect to see stronger coulombic forces with the electron for atoms with higher nuclear charge. If we compare the elements in the same row, then the electron affinity generally increases as the nuclear charge gets higher with decreasing atomic size. However, going down the groups of periodic table, the atomic size gets larger with the increasing nuclear charge, and because these two effects go in opposite directions, there is no certain way of predicting which element will have a stronger electron affinity.
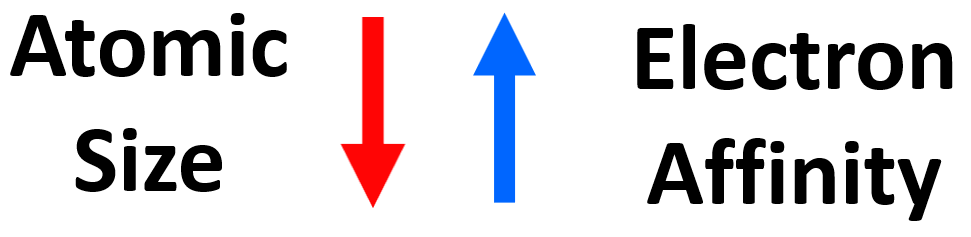
Let’s, for now, generalize that a smaller atomic size indicates a stronger electron affinity.
With this said, the electron affinity generally gets larger (more exothermic) as we moved to the right and up in the periodic table:
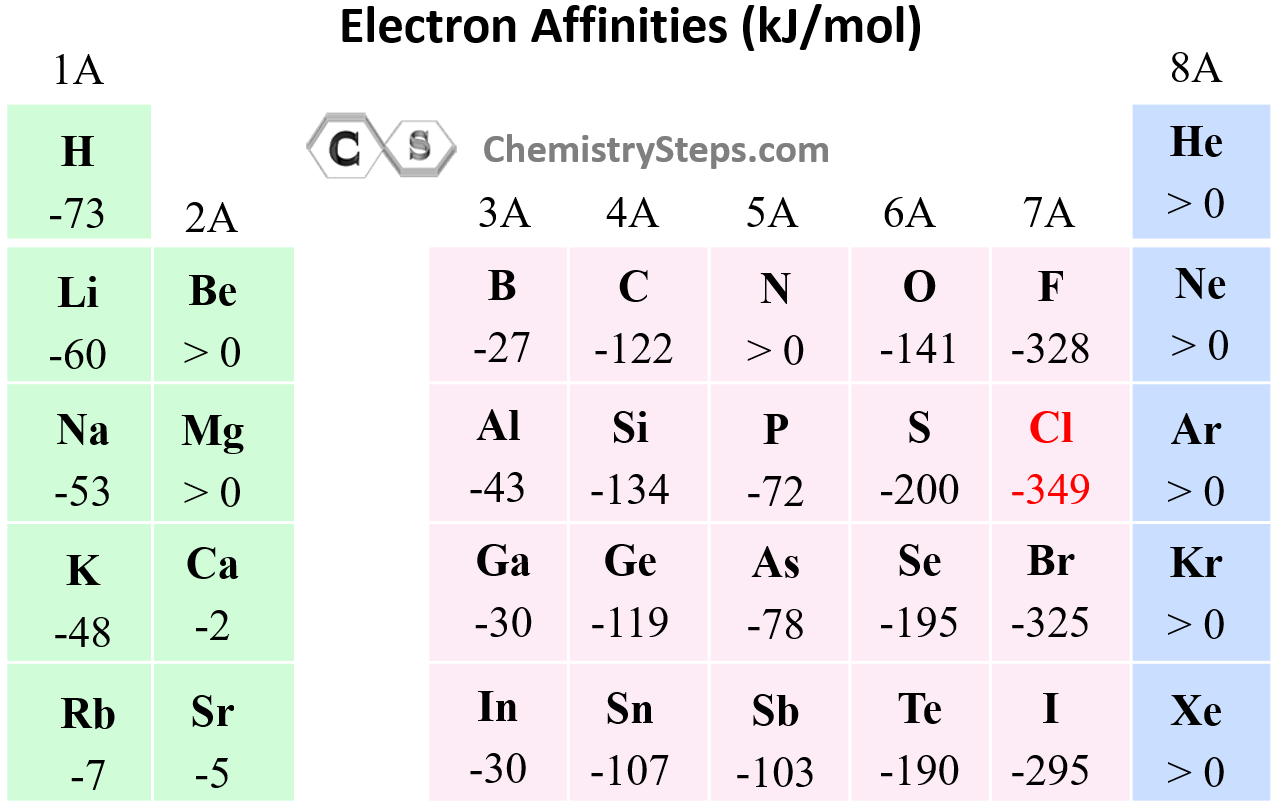
Electron Affinity of Halogens
Halogens have the greatest electron affinity, and this can be easily explained by looking at their valence shell electron configuration: ns2np5.
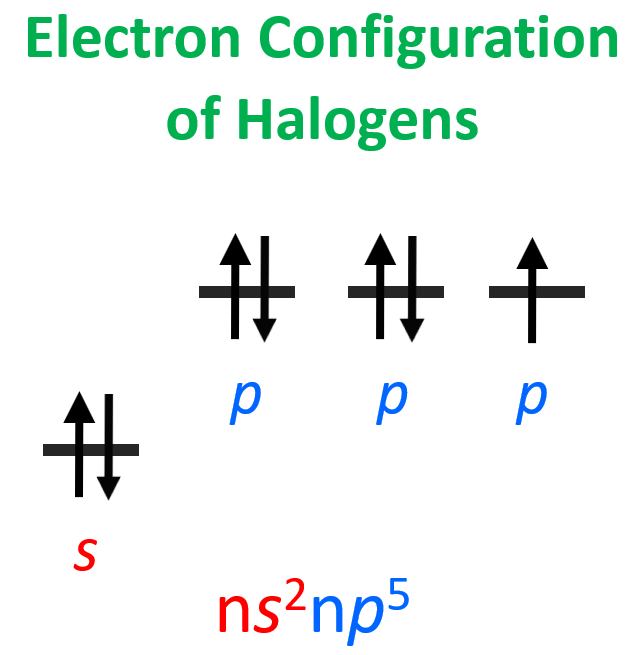
To attain electron configuration of a noble gas – that is having a complete valance shell, halogens need only one electron, and because this configuration is very stable, there is a significant heat release:
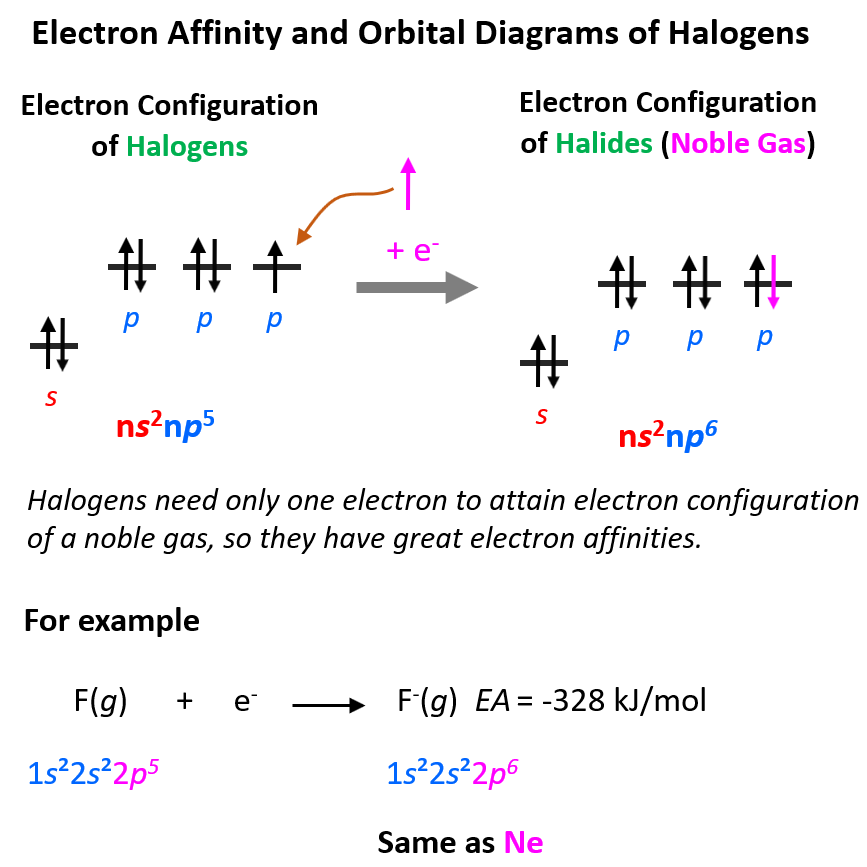
Notice that the trends are similar to those of electronegativity, although, the latter is referred to the tendency of the atom to pull the bonding electrons. This difference may be used to explain why chlorine has the greatest electron affinity.
Based on the atomic sizes, we would expect fluorine to have a stronger electron affinity than chlorine as it is smaller, and therefore, the incoming electron should experience stronger coulombic forces.
However, as we discussed, there should be an optimal ratio of the nuclear charge and atomic size to result in a stronger electron affinity. Now, because fluorine is too small, it is thought that accommodating so many electrons, when the atom is on its own, brings up the issue of repulsive forces between them. On the contrary, when fluorine is bonded to another atom, it is still sharing the electron pair which places them at a more optimal distance, thus making it the most electronegative element. The repulsive forces are not as pronounced in chlorine as it is a larger atom and turns to have the most optimal nuclear charge-to-distance ratio in isolated atoms which makes it the element with the greatest electron affinity.
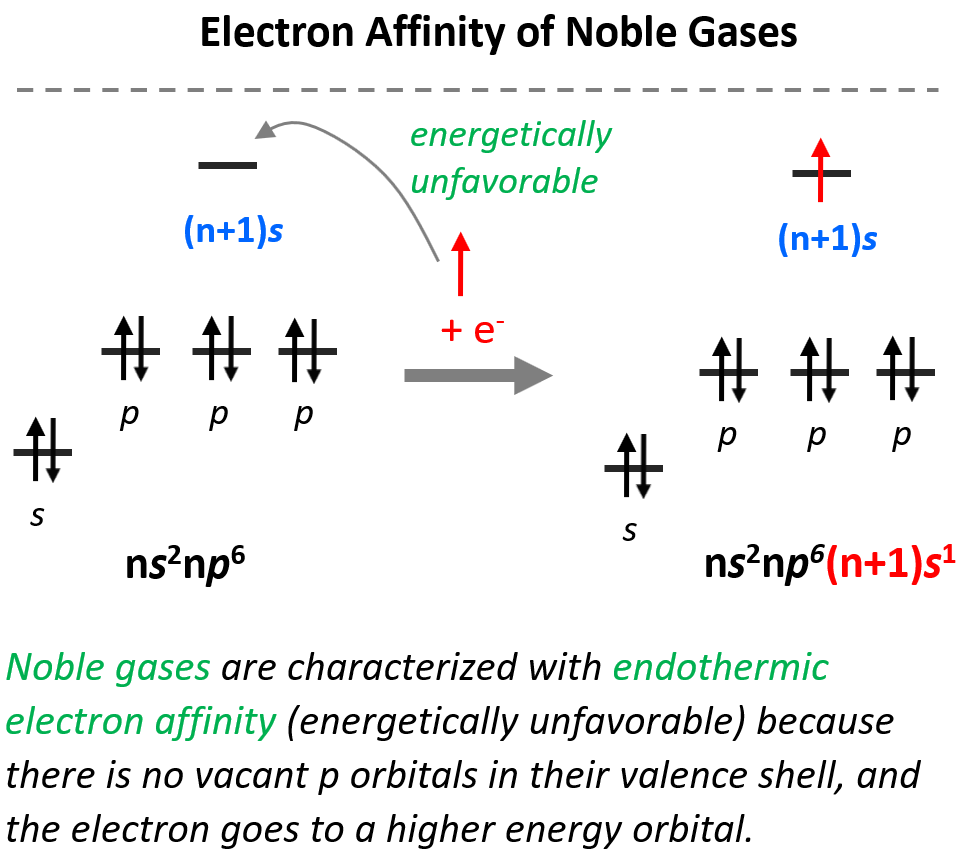
Electron Affinity of Noble Gases
Next to the right, we have the noble gases which are characterized by endothermic electron affinity because there are no vacant p orbitals in their valence shell, not even with one electron in it, and they do not “want” to add an electron to higher energy orbitals:
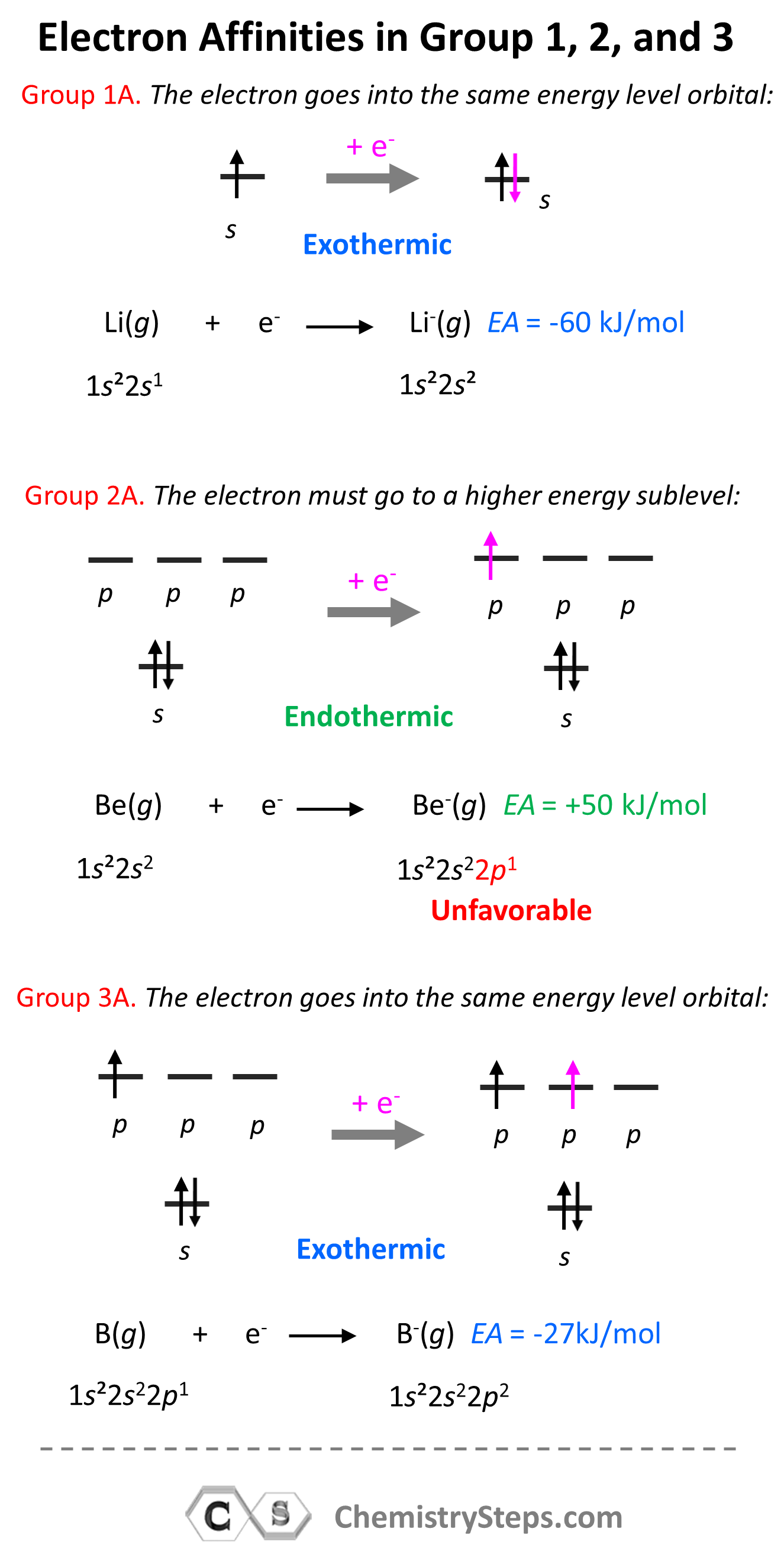
Electron Affinity in Groups 1A, 2A, and 3A
The same principle explains, for example, why elements in the second group have endothermic electron affinity, while those in the first and the third are happy to accept an electron.
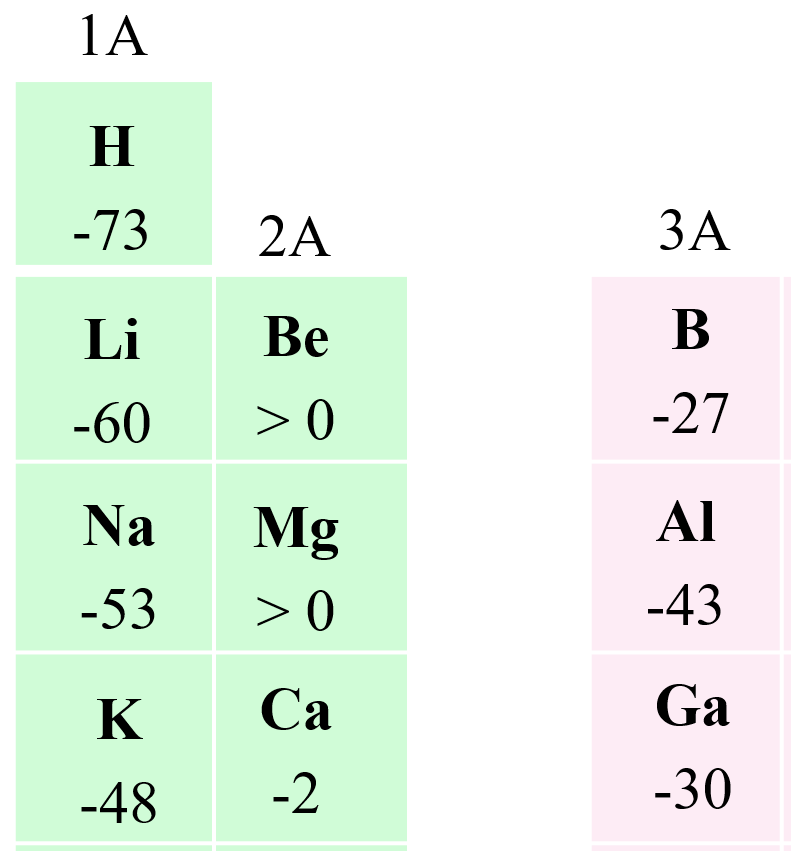
The second group elements have filled s orbitals and the coming electron would have to go to the p sublevel which is higher in energy than the s sublevel:
Electron Affinities of Carbon, Nitrogen, and Oxygen
Let’s also look at nitrogen which is between carbon and oxygen in the second row of the periodic table. How come adding an electron is exothermic for the carbon and oxygen, meaning they like to add an electron, but nitrogen is not as willing to do so?
To answer this question, we again need to look at the electron configuration and orbital diagrams of these elements. Carbon has an empty p orbital and adding an electron to it is energetically favorable – exothermic electron affinity. Nitrogen, on the other hand, has one electron in each p orbital, and adding another one to them pairs the electrons which is energetically unfavorable (recall the Hund’s rule):
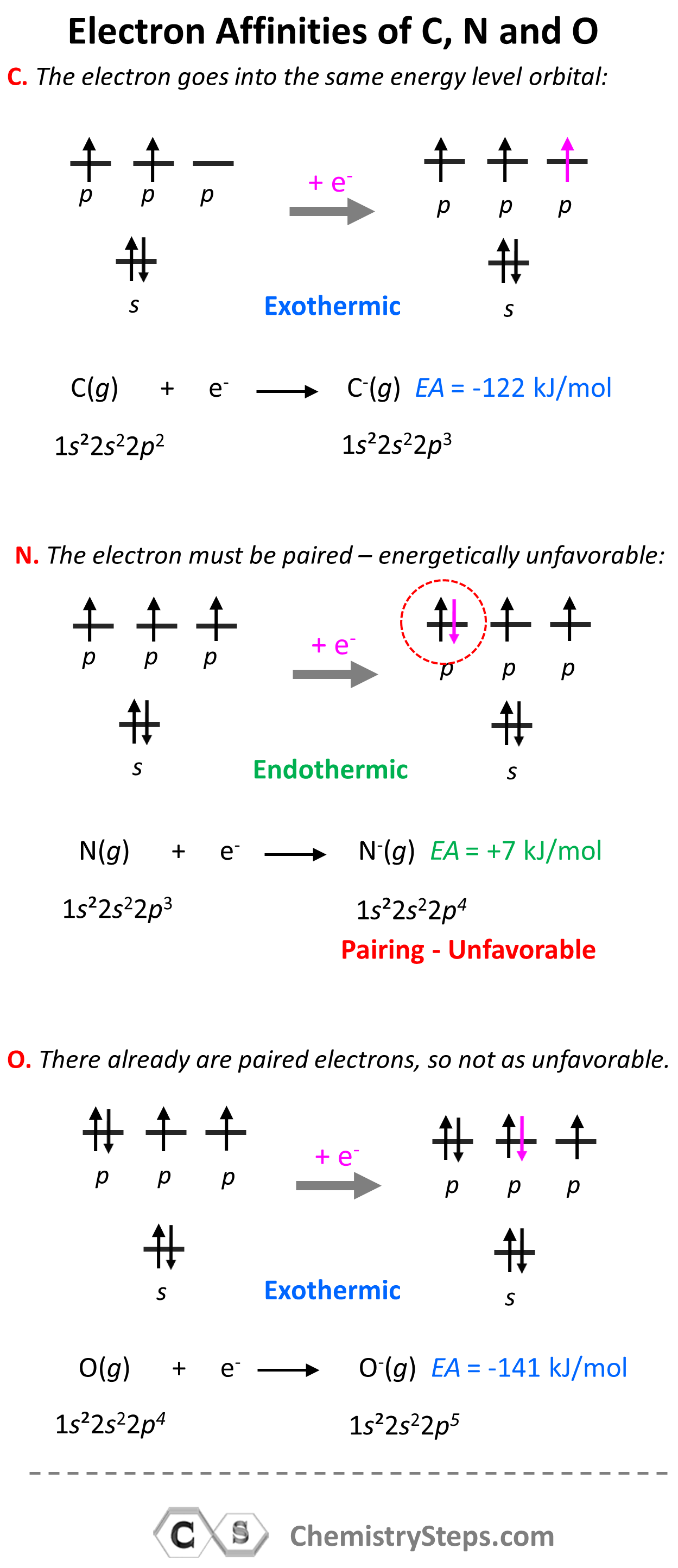
Now, you may wonder how come oxygen then likes to add an electron and pair it with another one in a p orbital. The reason is oxygen has a higher nuclear charge and smaller atomic size than nitrogen, besides, it already has a paired electron in the p orbital.
To summarize the trends in electron affinity, perhaps the most important is to remember that it becomes more negative (adding an electron becomes more exothermic) as we move to the right in a row of the periodic table. For some groups, we can also notice this going up, however, there are many exceptions, and it is always a good idea to consider the electron configurations.
Check this 95-question, Multiple-Choice Quiz on the Electronic Structure of Atoms including questions on properties of light such as wavelength, frequency, energy, quantum numbers, atomic orbitals, electron configurations, and more.
Check Also
- Atomic Orbitals
- Electron Configurations
- Electron Configurations of Ions
- Orbital Diagrams
- Aufbau’s Principle, Hund’s Rule, and Pauli’s Exclusion Principle
- Hund’s Rule
- Pauli Exclusion Principle
- Quantum Numbers (n, l, ml, ms)
- Bohr Model of the Hydrogen Atom
- Rydberg Formula
- The Photoelectric Effect
- Calculating The Energy of a Photon
- Ionization Energy
- Electron Affinity
- Energy, Wavelength, and Frequency Practice Problems

