The terms endothermic and exothermic are used to describe the heat transfer during different processes including chemical reactions. In general, the focus in this chapter is going to be on heat transfer because thermochemistry is the study of heat change in chemical reactions.
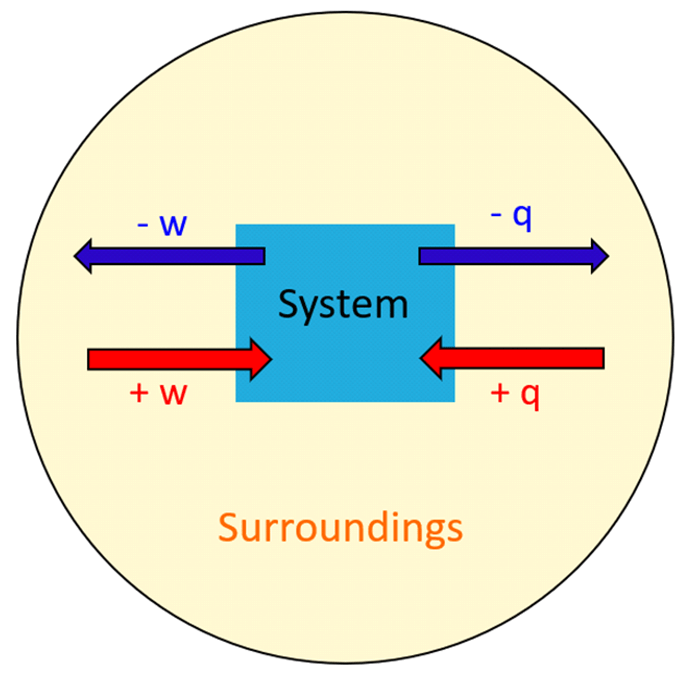
Remember, heat is the transfer of thermal energy between two bodies that are at different temperatures. So, in a broader perspective, it may be used for a system rather than an object. The system is essentially what we are interested in, while everything else is the surroundings.
In chemistry, the system is the reaction and all the changes in energy, heat, work or enthalpy are always looked at from the perspective of the system/reaction.
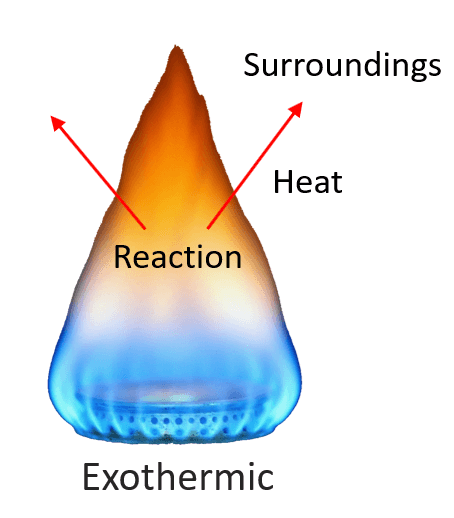
For example, the combustion reaction of methane (CH4), the main component of natural gas, releases a large amount of heat, and therefore, it is an exothermic reaction (exo means “out,” so heat flows out).
CH4(g) + 2O2(g) → CO2(g) + 2H2O(l) ΔHrxn = -891 kJ
The ΔH is the enthalpy change, which, remember is equal to the heat (q) of the reaction under constant pressure.
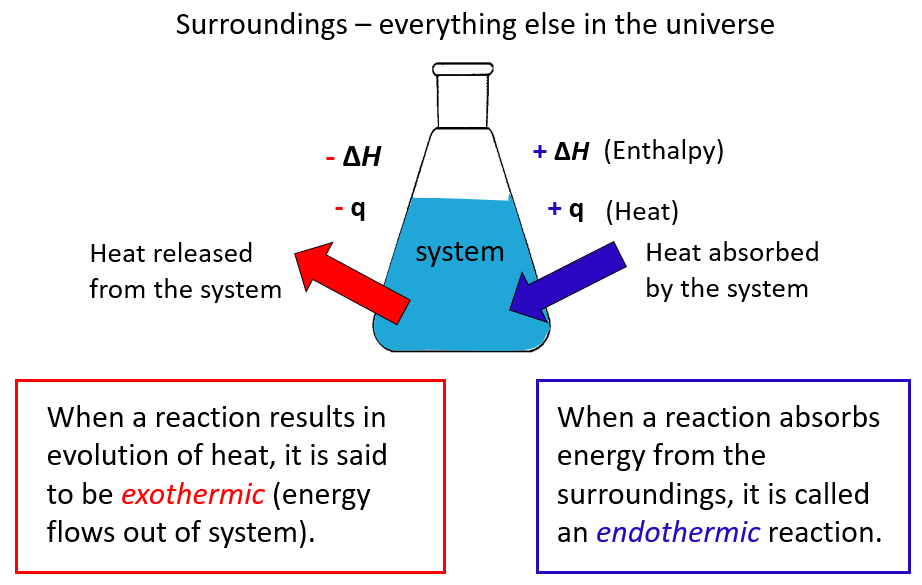
A chemical reaction that gives off heat to its surroundings is called an exothermic reaction. Exothermic reactions have a negative ΔH, since the enthalpy sum of the products is smaller than the one for products.
Endothermic reactions (endo means “within,” so heat flows in), on the other hand, absorb heat from surroundings and have a positive ΔH.
For example, the formation of nitrogen monoxide (NO) from oxygen and nitrogen gases is an endothermic reaction that occurs during thunderstorms at high temperatures:

N2(g) + O2(g) → 2NO(g) ΔHrxn = +180.5 kJ
Another example is the use of “instant cold” packs although these are not usually based on a chemical reaction, but rather on dissolving a salt such as ammonium nitrate (NH4NO3) or urea (CH₄N₂O). The salt is initially stored in a sealed plastic bag surrounded by water. When we break the container, the salt dissolved and dissociates in water breaking the ionic bonds which requires energy. This energy is obtained from the surroundings, from our body in this case.
To summarize, remember that:
- An endothermic reaction absorbs heat from the surroundings and has a positive ΔH
- An exothermic reaction releases heat to the surroundings and has a negative ΔH
- Under constant pressure, the ΔH of a reaction is the amount of heat (q) absorbed or evolved in the reaction.
Check Also
- Energy Related to Heat and Work
- Heat Capacity and Specific Heat
- Heat Capacity Practice Problems
- What is Enthalpy
- Constant-Pressure Calorimetry
- Bomb calorimeter – Constant Volume Calorimetry
- Stoichiometry and Enthalpy of Chemical Reactions
- Hess’s Law and Enthalpy of Reaction
- Hess’s Law Practice Problems
- Standard Enthalpies of Formation
- Enthalpy of Reaction from Enthalpies of Formation
- Thermochemistry Practice Problems