We have seen several examples demonstrating that water is an amphoteric compound as it can act both as an acid and as a base. For example, it reacts with HCl as a base, and with NH3 as an acid:
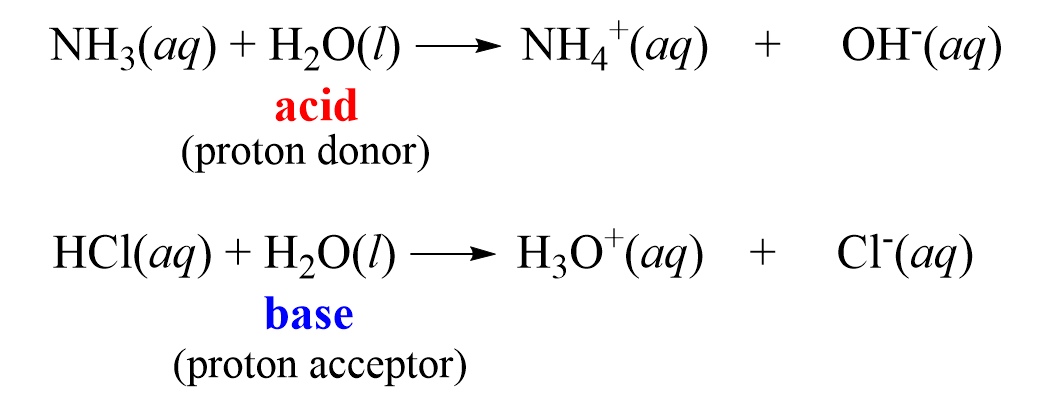
To generalize, we can say that in the presence of an acid, water acts as a base, while in the presence of a base, water acts as an acid.
Now, the question is can it react with itself? Can one water molecule be the acid and donate a proton to another one serving as the base?
The answer is yes, it can, and this reaction indeed occurs:
H2O(l) + H2O(l) ⇆ H3O+(aq) + OH–(aq)
This is called the autoionization or dissociation of water which, in a simpler form, can be shown as:
H2O(l) ⇆ H+(aq) + OH–(aq)
The equilibrium constant expression for this reaction is:
\[K\, = \,{\mkern 1mu} \frac{{[{{\rm{H}}^{\rm{ + }}}][{\rm{O}}{{\rm{H}}^{\rm{ – }}}]}}{{[{{\rm{H}}_{\rm{2}}}{\rm{O}}]}}\; = \;\frac{{[{{\rm{H}}_{\rm{3}}}{{\rm{O}}^{\rm{ + }}}][{\rm{O}}{{\rm{H}}^{\rm{ – }}}]}}{{[{{\rm{H}}_{\rm{2}}}{\rm{O}}]}}\]
It is determined that the concentration of water is constant (55.5 mol/l), so it is omitted from the expression, and the equilibrium constant becomes Kw which is equal to :
This equilibrium constant is called the ion product constant (dissociation constant) of water (Kw).
What is important is that the product of [H+] and [OH–] is constant and equals to 10-14 at 25 oC:
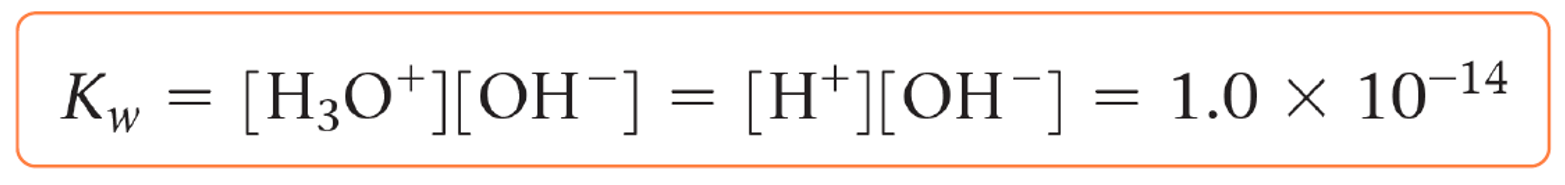
In pure water, the concentrations of H+ and OH– ions are equal, so by taking the square root of Kw, the concentration of H+ and OH– ions in water are found to be:
\[{\rm{[}}{{\rm{H}}^{\rm{ + }}}{\rm{]}}\; = \;{\rm{[O}}{{\rm{H}}^{\rm{ – }}}{\rm{]}}\;{\rm{ = }}\;\sqrt {{K_{\rm{w}}}} \; = \;1.0\, \times \,{10^{ – 7}}\,M\]
When [H+] = [OH–], we have a neutral solution, when [H+] > [OH–] the solution becomes acidic, and if [H+] < [OH–], then it is a basic solution.
We can change the concentrations of H+ and OH– but because they are correlated and their product is constant, we cannot do it independently. For example, if we increase [H+], the [OH–] will decrease and vice versa.
Given the [H+] or [OH-], we can calculate the other one.
For example, if [H+] = 10-3, we can determine the [OH–] using the expression for Kw:
Kw = [H+][OH–], therefore,
\[{\rm{[O}}{{\rm{H}}^{\rm{ – }}}{\rm{]}}\;{\rm{ = }}\;\frac{{\sqrt {{K_{\rm{w}}}} \;}}{{{\rm{[}}{{\rm{H}}^{\rm{ + }}}{\rm{]}}\;}}\; = \;\frac{{{{10}^{ – 14}}\;}}{{{{10}^{ – 3}}\;}}\, = 1.0\, \times \,{10^{ – 11}}\,M\]
So, this is an acidic solution because [H+] > [OH–].
In the next article, we will talk about the pH scale as an indicator of the acidity or basicity of the solution.
Check Also
- Definitions of Acids and Bases
- Acid-Base Reactions
- Acid-Base Titrations
- Conjugate Acid and Conjugate Base
- The pH and Acidity
- Acid Strength, Ka, and pKa
- Base Strength, Kb and pKb
- Ka, pKa, Kb, and pKb Relationship
- The pH of a Strong Acid and Base
- pH + pOH = 14
- The pH of a Weak Acid
- The pH of a Weak Base
- ThepH of Polyprotic Acids
- The acidity of a Salt Solution
- The pH of a Salt Solution
- The pH of Salts With Acidic Cations and Basic Anions
- Acids and Bases Practice Problems
