Because the concentrations of H+ and OH– ions in aqueous solutions are usually very small, to specify the acidity of a solution with more convenient numbers, a quantity called pH is used.
The pH is defined as the negative logarithm of proton/hydronium ion concentration:
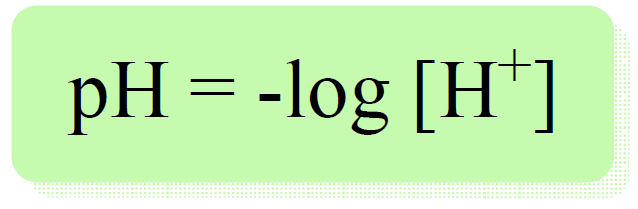
For example, if [H+] = 10-4 mol/l, the pH will be:
pH = -log 10-4 = 4 (acidic)
How do we know it is an acidic solution?
Remember, from the dissociation of water that Kw = [H+][OH–] = 10-14 and if [H+] > 10-7 M, then the solution is acidic, and if [H+] < 10-7 M, we have a basic solution.
So, let’s say we have a basic solution with [OH–] = 10-5. The [H+] can be calculated from Kw:
\[{\rm{[}}{{\rm{H}}^{\rm{ + }}}{\rm{]}}\;\;{\rm{ = }}\;\frac{{\sqrt {{K_{\rm{w}}}} \;}}{{{\rm{[O}}{{\rm{H}}^{\rm{ – }}}{\rm{]}}}}\; = \;\frac{{{{10}^{ – 14}}\;}}{{{{10}^{ – 5}}\;}}\, = 1.0\, \times \,{10^{ – 9}}\,M\]
And the pH, therefore, is:
pH = -log 10-9 = 9 (basic)
Now, for a neutral solution, the pH is 7 because a solution is neutral when [H+] = [OH–] = 10-7 M, and the pH in this case is:
pH = -log 10-7 = 7 (neutral)
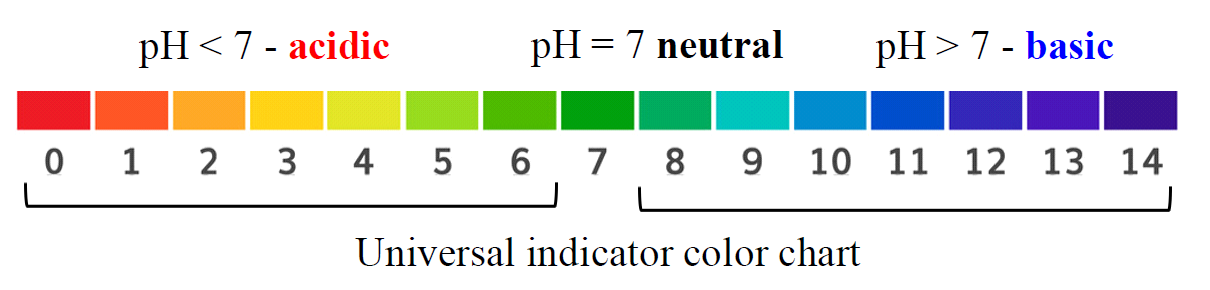
So, the pattern is; if pH < 7, the solution is acidic, if pH = 7, the solution is neutral, and if pH > 7, we have a basic solution.
The pH scale goes from 0 to 14. The smaller the pH, the more acidic the solution.
The pOH and Basicity
Although, not as commonly used, the pOH values also describe the acidity/basicity of the solution. It is again the negative log which is a convenient method for expressing small numbers.
pOH = – log [OH–]
Example:
Calculate the pOH of a solution with [OH–] = 10-2 (basic).
pOH = – log [OH–] = -log 10-2 = 2
Like in the pH scale, a solution is neutral if pOH = 7. A pOH less than 7 is basic and a pOH greater than 7 is acidic.
In the next few post, we will discuss the relationship between pH and pOH and how it is used to calculate the pH and pOH from one another.
Check Also
- Definitions of Acids and Bases
- Acid-Base Reactions
- Acid-Base Titrations
- Conjugate Acid and Conjugate Base
- Autoionization of Water and Kw
- The pH and Acidity
- Acid Strength, Ka, and pKa
- Base Strength, Kb and pKb
- Ka, pKa, Kb, and pKb Relationship
- The pH of a Strong Acid and Base
- pH + pOH = 14
- The pH of a Weak Acid
- The pH of a Weak Base
- ThepH of Polyprotic Acids
- The acidity of a Salt Solution
- The pH of a Salt Solution
- The pH of Salts With Acidic Cations and Basic Anions
- Acids and Bases Practice Problems
