The first thing we need to do when drawing a Lewis structure is determine the total number of valence electrons in the molecule. Remember, valence electrons are those in the outermost principal energy level. For example: Na – 1s22s22p63s1, Cl – 1s22s22p63s23p5
The number of valence electrons, for main group elements, corresponds to their group number in the periodic table:
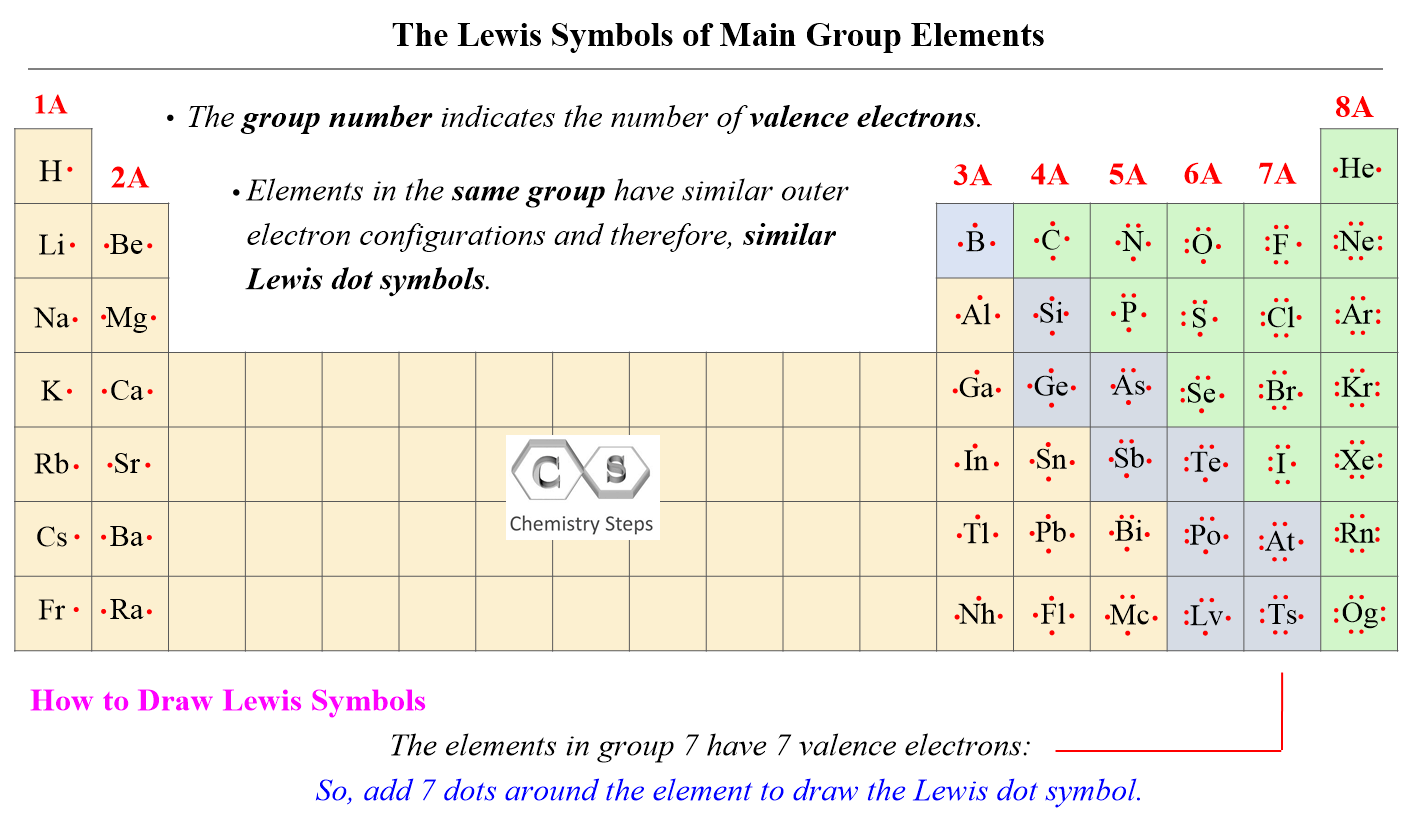
For d block elements, the outermost d electrons are also counted as valence electrons (ns + (n-1)d). For example, iron has eight valence electrons: Fe – 1s22s22p63s23p64s23d6.
So, nitrogen is in group 5A, and therefore, it has 5 valence electrons, thus N2 has 10 valence electrons.
Next, we need to connect the atoms in the correct order and add the electrons as bonds and lone pairs.
In short, these are the steps you need to follow for drawing a Lewis structure:
1. Write the correct skeletal structure for the molecule.
* Hydrogen atoms are always terminal (only one bond)
* Put more electronegative elements in terminal positions
2. Sum the valence electrons from all the atoms.
3. Use a pair of electrons to form a bond between each pair of bound atoms.
4. Add the remaining electrons to satisfy the octet for a more electronegative atom first.
5. If any atoms lack an octet, make a double or triple bond to give them an octet.
Out of the 10 electrons, two are going to make a bond between the two atoms:
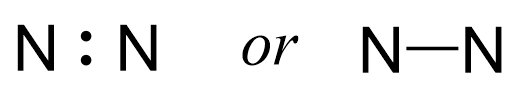
Notice that the bonds can be shown as a line and in fact, this is the most common way of showing structures once we learn the principles of Lewis structures.
We now have 8 valence electrons left, and we add them to each nitrogen as two lone pairs:
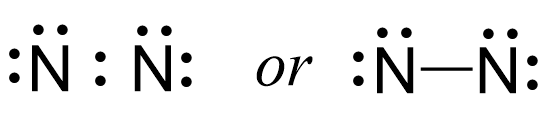
Next, check if the atoms have octet. Each nitrogen is surrounded by six electrons, and what we do to make an octet is move one of each lone pairs to make a triple bond:
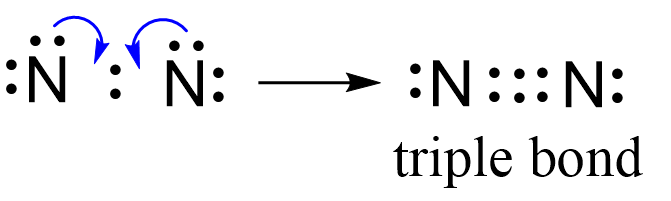
And now the nitrogen have complete octet:
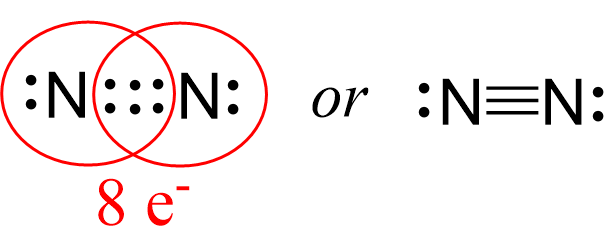
Lastly, check for formal charges. Remember, less charges is better and if there is a way neutralizing the charges by making more bonds, we do it unless it violates the octet for the second-row elements.
We can use this formula for calculating formal charges:
FC= V – (N + B)
Where:
V – number of valence electrons
N – number of nonbonding electrons
B – number of bonds
So, the formal charge of the oxygen will be
FC (N) = 5 – (2 + 3) = 0
Both nitrogen have an octet and there is no formal, so this is the correct Lewis structure of nitrogen gas.
Check this 90-question, Multiple-Choice Quiz on Chemical Bonding:
Check Also
- Lewis Dot Symbols
- The Ionic Bond
- The Covalent Bond
- Sigma and Pi Bonds
- Electronegativity and Bond Polarity
- The Octet Rule
- Formal Charges
- Lewis Structures and the Octet Rule
- Lewis Structures Practice Problems
- Resonance Structures

