To convert the grams to moles, we need to know the molar mass of the substance. Remember, molar mass (M) is the mass of one mole of atoms/molecules/ions expressed in g/mol. To calculate it we are always going to need a periodic table. So, how do we find the molar mass in the periodic table?
For example, there are two numbers given for potassium in the periodic table: 19 and 39.098. The smaller one is the atomic number which shows the number of protons, and the larger one is the average atomic mass:

What’s important for today’s topic is that numerically, the molar mass is equal to the average atomic mass of the given atom, so we can look it up in the periodic table. For potassium then, it is 39.098 g/mol which means one mole of potassium atoms weighs 39.098 g. And we can do this for all the elements in the periodic table.
Converting Mass to Moles
To calculate the moles from a given mass (m), the molar mass of the component is used. The given mass is the mass of the sample, and it can be any number, for example, we can have 10 g of salt, 15 g, or 100 g. The molar mass, on the other hand, is a constant number for a given atom or a molecule as it is for a specific amount of it.
So, to calculate the moles from a given mass, we make a conversion factor correlating 1 mole with the molar of the given component.
For example, how many moles of sulfur are there in a 16.2 g sample?
The molar mass of sulfur is 32.1 g/mol, and therefore, the conversion factors are:
\[\frac{{{\rm{1}}\;{\rm{mol}}\;{\rm{S}}}}{{{\rm{32}}{\rm{.1}}\;{\rm{g}}\,{\rm{S}}}}\;\,and\,\,\frac{{{\rm{32}}{\rm{.1}}\;{\rm{g}}\,{\rm{S}}}}{{{\rm{1}}\;{\rm{mol}}\;{\rm{S}}}}\]
These two simply indicate that 1 mol of S weighs 32.1 g, which is the molar mass of the sulfur which we find it in the periodic table.
Now, to calculate the molar mass, we need to choose the correct conversion factor. Remember, the idea is to choose the one that allows to cancel the gram units. So, it should be the one where the grams are in the denominator.
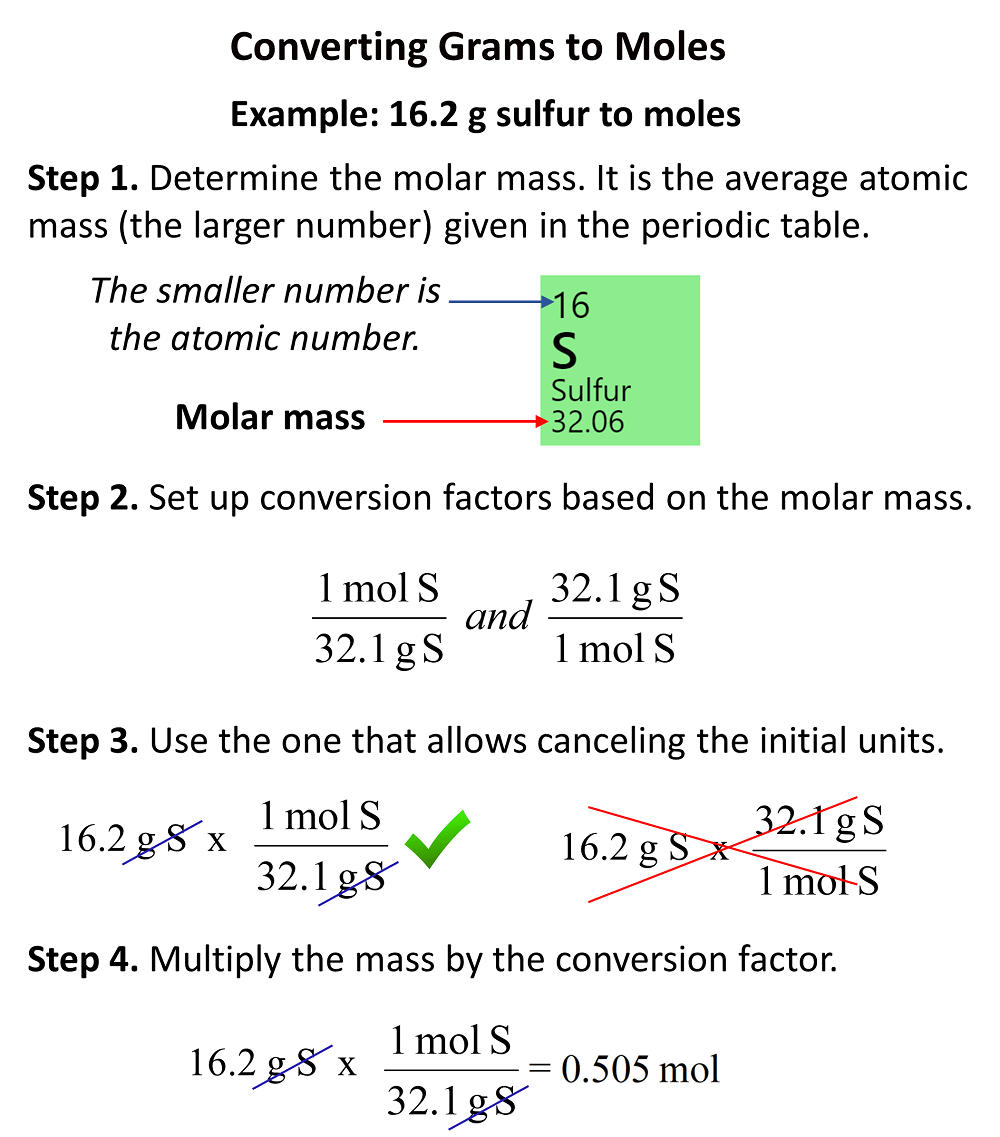
Once you determine the correct conversion factor, multiply the numbers and cancel out the units:
There is also a formula correlating the number of moles with the molar mass (M) and the given mass (m). So, you can also use this formula when converting between mass, molar mass, and moles:
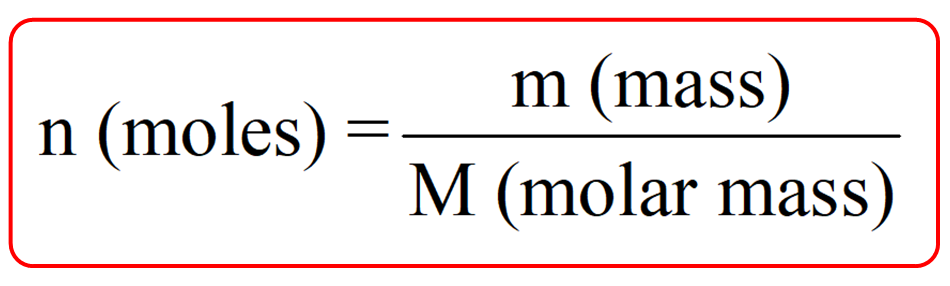
For our example of 13.2 g sulfur, we’d have:
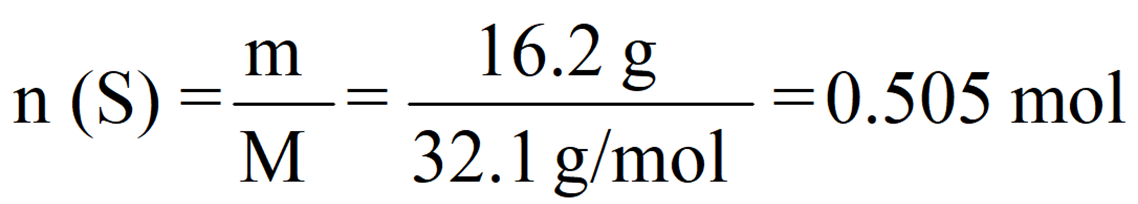
Notice that the exact answer in the calculator is 0.5046728 and we round it off to three significant figures because both initial numbers contain three significant figures.
Moles of Molecules from the Mass
To calculate the moles of a molecule, from the given mass, we are following the same steps as we did for the atom. The only difference is that the molar mass of the molecule is not going to be given in the periodic table, so you will need to calculate it first.
To calculate the molar mass of a molecule, we add the molar mass of each constituent atom by the corresponding subscript.
For example, the molar mass of water would be:
M (H2O) = 2 x 1.0 + 16.0 = 18.0 g/mol
If the formula of the molecule is not given, you will need to first determine it.
For example, determine the molar mass of phosphorous pentachloride.
The formula of phosphorous pentachloride is PCl5. Go over naming covalent compounds to refresh some of the concepts here.
To calculate the molar mass of PCl5, we multiply the molar mass of each element by its subscript:
M (PCl5) = M (P) + 5x M (Cl)
M (PCl5) = 30.1 + 5 x 35.5 = 207.6 g/mol
Another example: what is the molar mass of aluminum sulfate?
First, we know that the aluminum ion has a 3+ charge. You need to know the common polyatomic ions from where we find that sulfate is the SO42- ion. Next, we write the ions next to each other and add the correct subscripts:
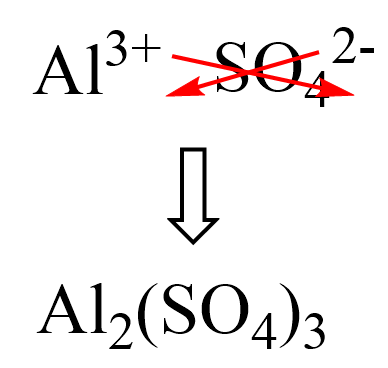
And now, we multiply the molar mass of each element by its subscript. Remember, the number outside of parenthesis applies to all the elements in it.
M (Al2(SO4)3) = 2 x M (Al) + 3 x M (S) + 3 x 4 x M (O)
M (Al2(SO4)3) = 2 x 27.0 + 3 x 32.1 + 3 x 4 x 16.0 = 342.3 g/mol
We have calculated the molar mass of Al2(SO4)3, so let’s say we need to determine the number of moles in a 78.1 g sample.
The conversion factors are:
\[\frac{{{\rm{1}}\;{\rm{mol}}\;{\rm{A}}{{\rm{l}}_{\rm{2}}}{{{\rm{(S}}{{\rm{O}}_{\rm{4}}}{\rm{)}}}_{\rm{3}}}}}{{342.3\;{\rm{g}}\,{\rm{A}}{{\rm{l}}_{\rm{2}}}{{{\rm{(S}}{{\rm{O}}_{\rm{4}}}{\rm{)}}}_{\rm{3}}}}}\;\,or\,\,\frac{{342.3\;{\rm{g}}\,{\rm{A}}{{\rm{l}}_{\rm{2}}}{{{\rm{(S}}{{\rm{O}}_{\rm{4}}}{\rm{)}}}_{\rm{3}}}}}{{{\rm{1}}\;{\rm{mol}}\;{\rm{A}}{{\rm{l}}_{\rm{2}}}{{{\rm{(S}}{{\rm{O}}_{\rm{4}}}{\rm{)}}}_{\rm{3}}}}}\]
We are starting with grams, and therefore, the first conversion factor is correct as it will allow us to cancel the gram units:
\[{\rm{n}}\,{\rm{ = }}\,{\rm{78}}{\rm{.1}}\,\cancel{{{\rm{g}}\,{\rm{A}}{{\rm{l}}_{\rm{2}}}{{{\rm{(S}}{{\rm{O}}_{\rm{4}}}{\rm{)}}}_{\rm{3}}}}}{\rm{ \times }}\,\frac{{{\rm{1}}\;{\rm{mol}}\;{\rm{A}}{{\rm{l}}_{\rm{2}}}{{{\rm{(S}}{{\rm{O}}_{\rm{4}}}{\rm{)}}}_{\rm{3}}}}}{{{\rm{342}}{\rm{.3}}\;\cancel{{{\rm{g}}\,{\rm{A}}{{\rm{l}}_{\rm{2}}}{{{\rm{(S}}{{\rm{O}}_{\rm{4}}}{\rm{)}}}_{\rm{3}}}}}}}\,{\rm{ = }}\,{\rm{0}}{\rm{.228}}\;{\rm{mol}}\,{\rm{A}}{{\rm{l}}_{\rm{2}}}{{\rm{(S}}{{\rm{O}}_{\rm{4}}}{\rm{)}}_{\rm{3}}}\]
Moles of a Specific Atom
Sometimes you may be asked to calculate the moles of a specific atom given the mass of a compound. For example, how many moles of nitrogen atoms are there in 46.0 g sample of N2O3?
What you need to do here is determine first how many moles of that atom we have in mole of the compound. Remember, this we are doing based on the chemical formula. Every 1 mole of N2O3 contains 2 moles of N because every molecule of N2O3 contains 2 atoms of nitrogen. Therefore, once we calculate the moles of the oxide, we can multiply it by 2 to determine the moles of the nitrogen.
\[{\rm{n}}\,{\rm{(}}{{\rm{N}}_{\rm{2}}}{{\rm{O}}_{\rm{3}}}{\rm{)}}\;{\rm{ = }}\,{\rm{46}}{\rm{.0}}\,\cancel{{{\rm{g}}\,{{\rm{N}}_{\rm{2}}}{{\rm{O}}_{\rm{3}}}}}{\rm{ \times }}\,\frac{{{\rm{1}}\;{\rm{mol}}\;{{\rm{N}}_{\rm{2}}}{{\rm{O}}_{\rm{3}}}}}{{{\rm{76}}{\rm{.01}}\;\cancel{{{\rm{g}}\,{{\rm{N}}_{\rm{2}}}{{\rm{O}}_{\rm{3}}}}}}}\,{\rm{ = }}\,{\rm{0}}{\rm{.605}}\;{\rm{mol}}\,{{\rm{N}}_{\rm{2}}}{{\rm{O}}_{\rm{3}}}\]
Next, we multiply this by 2:
n (N) = 2 n (N2O3) = 2 x 0.605 mol = 1.21 mol
We can also do this calculation in one step using conversion factors:
\[{\rm{n}}\,{\rm{(N)}}\;{\rm{ = }}\,{\rm{46}}{\rm{.0}}\,\cancel{{{\rm{g}}\,{{\rm{N}}_{\rm{2}}}{{\rm{O}}_{\rm{3}}}}}{\rm{ \times }}\,\frac{{{\rm{1}}\;\cancel{{{\rm{mol}}\;{{\rm{N}}_{\rm{2}}}{{\rm{O}}_{\rm{3}}}}}}}{{{\rm{76}}{\rm{.01}}\;\cancel{{{\rm{g}}\,{{\rm{N}}_{\rm{2}}}{{\rm{O}}_{\rm{3}}}}}}}\,{\rm{ \times }}\,\frac{{{\rm{2}}\;{\rm{mol}}\;{\rm{N}}}}{{{\rm{1}}\;\cancel{{{\rm{mol}}\;{{\rm{N}}_{\rm{2}}}{{\rm{O}}_{\rm{3}}}}}}}{\rm{ = }}\,{\rm{1}}{\rm{.21}}\;{\rm{mol}}\,{\rm{N}}\]
Converting Moles to Mass
If the question asks to calculate the mass from the moles, we will need to use the other conversion factor so that the moles now can be canceled.
For example, how many moles of carbon are there in a 0.048 mol sample?
The molar mass of carbon is 12.0 g/mol, so the conversion factors are:
\[\frac{{{\rm{1}}\;{\rm{mol}}\;{\rm{C}}}}{{{\rm{12}}{\rm{.0}}\;{\rm{g}}\,{\rm{S}}}}\;\,and\,\,\frac{{{\rm{12}}{\rm{.0}}\;{\rm{g}}\,{\rm{C}}}}{{{\rm{1}}\;{\rm{mol}}\;{\rm{C}}}}\]
Using the second conversion factor, we can calculate the mass as:
\[{\rm{m}}\;{\rm{(C)}}\,{\rm{ = }}\;{\rm{0}}{\rm{.048}}\;\cancel{{{\rm{mol}}\,{\rm{C}}}}\,{\rm{ \times }}\,\frac{{{\rm{12}}{\rm{.0}}\;{\rm{g}}\,{\rm{C}}}}{{{\rm{1}}\;\cancel{{{\rm{mol}}\;{\rm{C}}}}}}\;{\rm{ = }}\;{\rm{0}}{\rm{.576}}\,{\rm{g}}\]
Practice
Calculating Moles from Mass
Determine the number of moles in 59.7 grams of Al.
Determine the number of moles in 2.41 grams of FeO.
Calculate the number of moles in 0.647 grams of Al2O3.
Determine the number of moles in 3.56 grams of Mg(OH)2.
Determine the number of moles in 0.385 grams of N2O3.
Determine the number of moles in 165 grams of CaSO4.
Calculate the molar mass of N2O4 and determine how many moles of it are in a 23.9 g sample.
Calculate the number of moles in 165 grams of C3H6O.
Determine the number of moles in 452 grams of Co(NO3)3.
Calculating Mass from Moles
Calculate the mass in grams of 0.598 moles of Fe.
Calculate the mass in grams of 0.168 moles of NO.
Calculate the mass in grams of 0.987 moles of (NH4)2S.
Calculate the mass in grams of 6.81 moles of Al2(SO4)3.
Calculate the mass in grams of 2.64 moles of methanol, CH3OH.
Calculate the mass in grams of 9.42 moles of NiCl2·6H2O.
Calculating the Number of Molecules from the Moles
How many molecules are there in a 0.487 mol sample of PCl5?
How many molecules (formula units) are there in a 5.84 mol sample of Na2SO3.
How many molecules of sucrose, C12H22O11 are there in a 0.684 mol sample?
Calculate the number of molecules in a 3.25-mol sample of propane, C3H8.
How many moles is 5.80 x 1025 molecules of POCl3?
Calculating the Number of Molecules from the Mass
How many molecules are there in a 5.12-g sample of K2O?
How many molecules of glucose, C6H12O6 are there in a 35.0 g sample?
Calculate the number of molecules of butane, C4H10, in its 2.40-gram sample.
How many Ethylene, C2H4 molecules are present in a 46.2 g sample? The molar mass of C2H4 is 28.1 g/mol.
Calculating the Number of Atoms
Calculate the number of atoms in a 2.56-g sample of Ca.
How many carbon atoms are there in a 0.590 mol sample of CCl4.
How many carbon atoms are there in a 0.964 mol sample of C2H6.
Which sample contains more Cl atoms: a) 1.25 moles of CH2Cl2 b) 2.15 moles of CH3Cl
The molecular formula of morphine is C17H19NO3. How many carbon atoms are in a 34.7-gram sample of morphine?
Isopropyl alcohol, also known as isopropanol, has found a widespread application in the preparation of pharmaceutical products. Answer the following questions considering that the molecular formula of isopropanol is C3H8O.
a) How many moles of C3H8O are contained in a 12.0 g sample of the alcohol?
b) How many molecules of C3H8O are contained in a 12.0 g sample of the alcohol?
c) How many atoms of oxygen are contained in a 12.0 g sample of the isopropyl alcohol (C3H8O)?
d) How many atoms of carbon are contained in a 12.0 g sample of the isopropyl alcohol (C3H8O)?
Check Also
- Subatomic particles and Isotopes
- Naming Monatomic and Polyatomic Ions
- Naming Ionic Compounds
- Naming Covalent Compounds
- Naming Acids and Bases
- Atomic and Molecular Masses
- The Mole and Molar Mass
- Percent Composition and Empirical Formula
- Stoichiometry of Chemical Reactions
- Limiting Reactant
- Limiting Reactant Practice Problems
- Reaction/Percent Yield
- Stoichiometry Practice Problems
