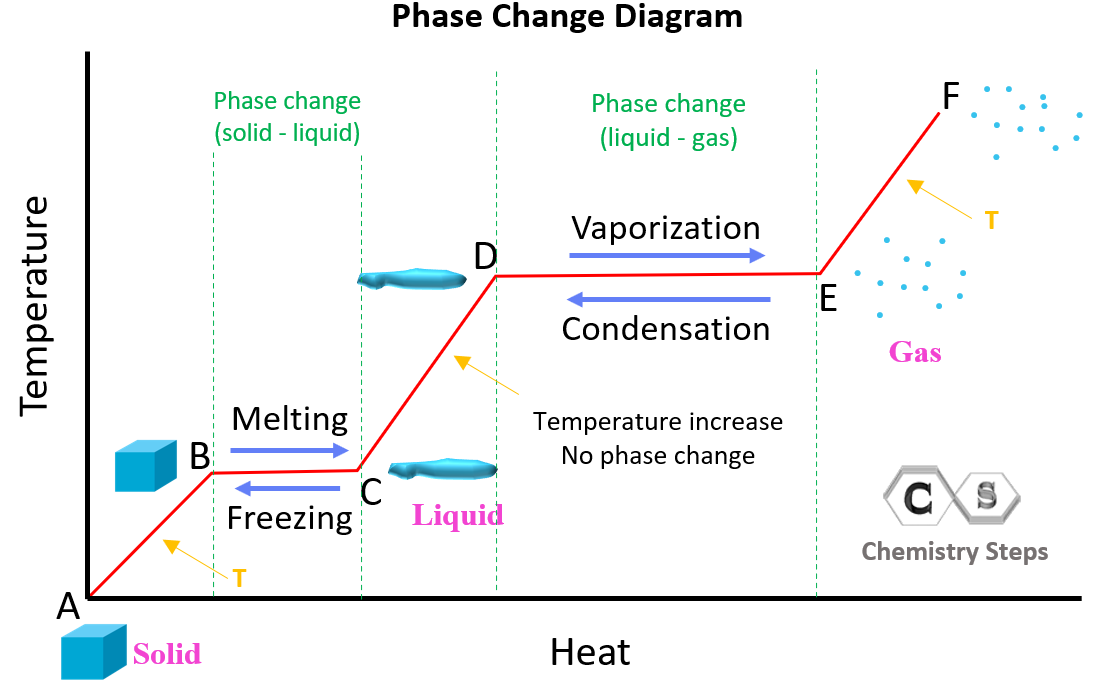
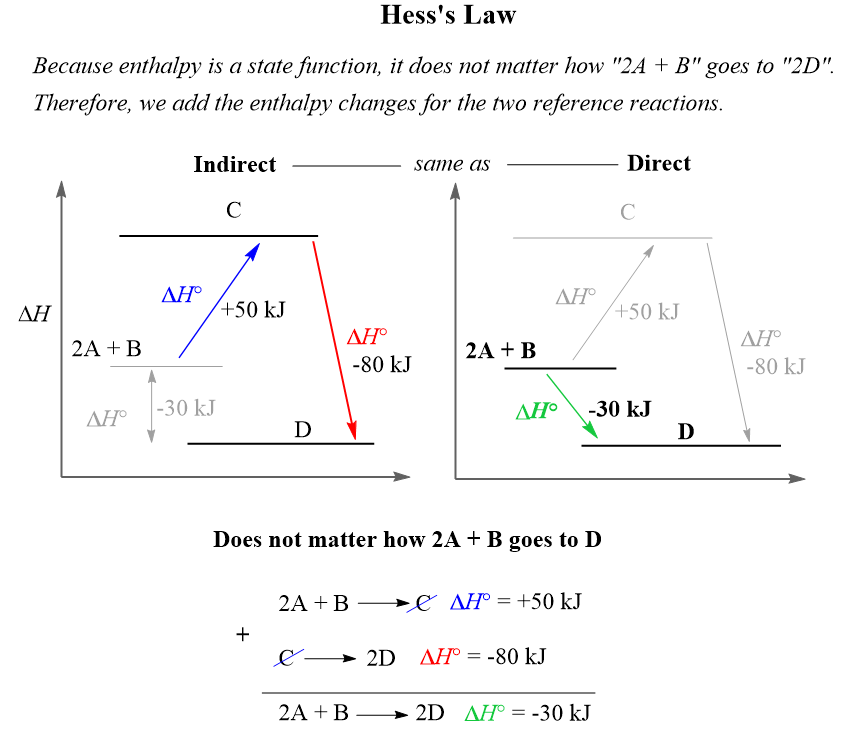
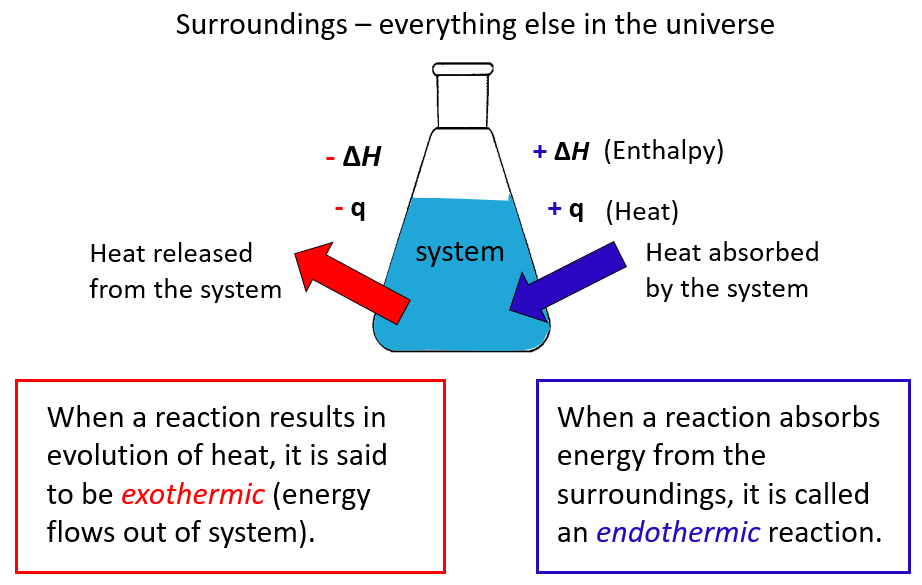
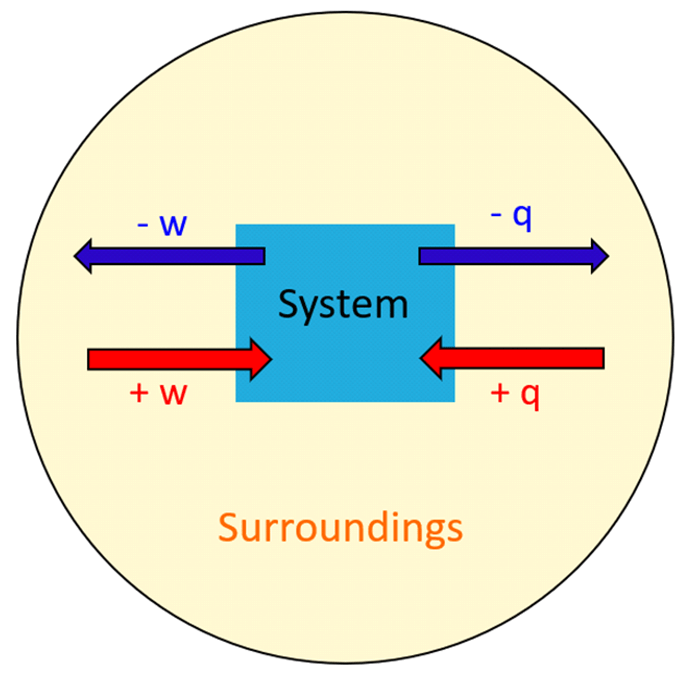
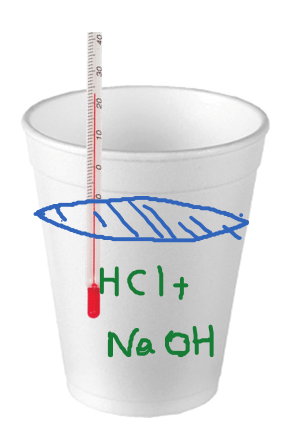
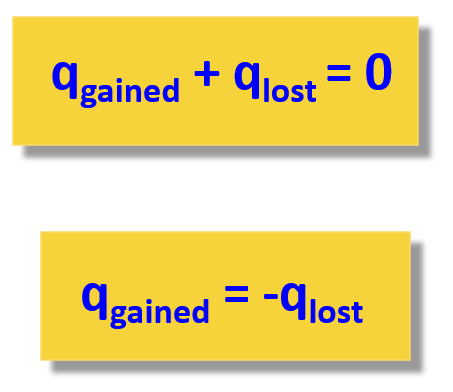
Heat and Phase Change Diagrams
In the previous post, we talked about Heat Capacity and Specific Heat. The formula we use to calculate the heat transferred between the objects is given as: By using this formula, we were able to calculate, for example, … Read more