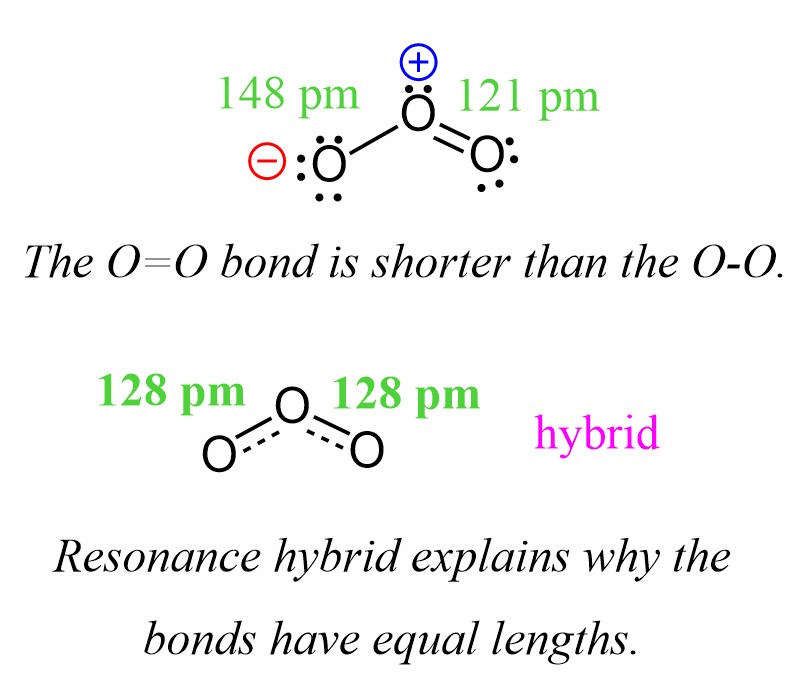
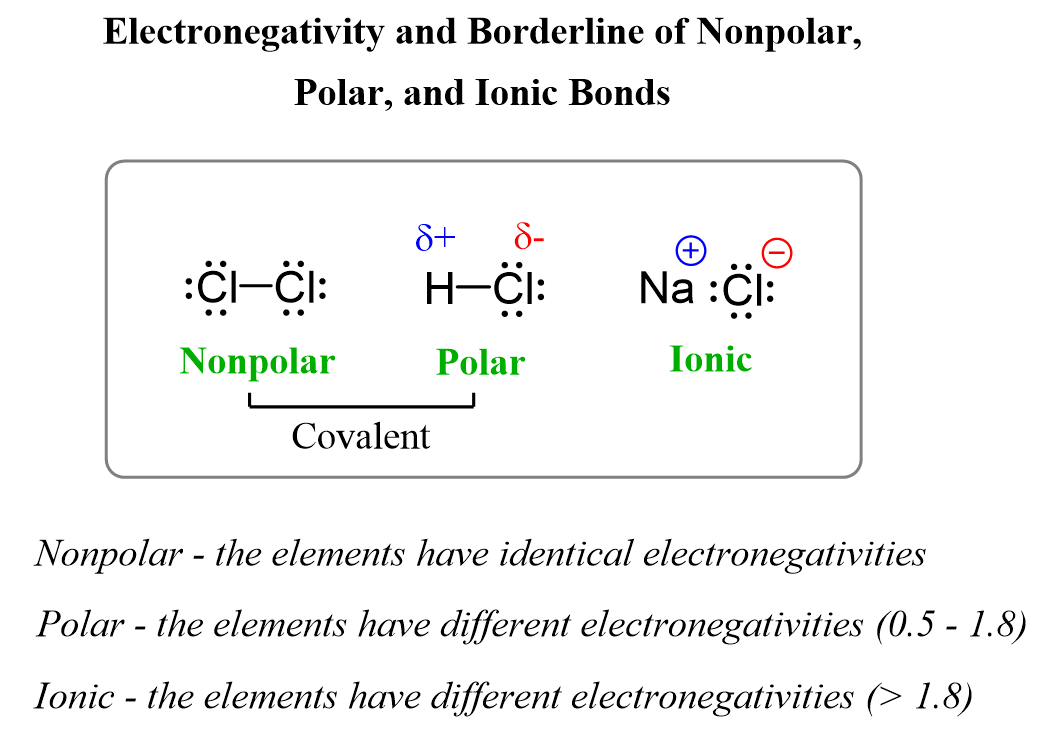
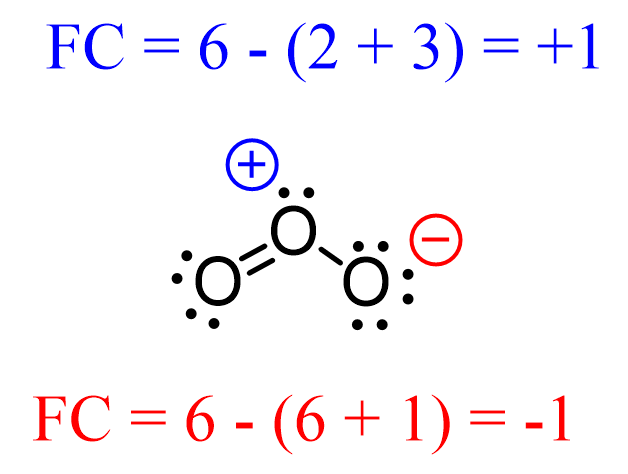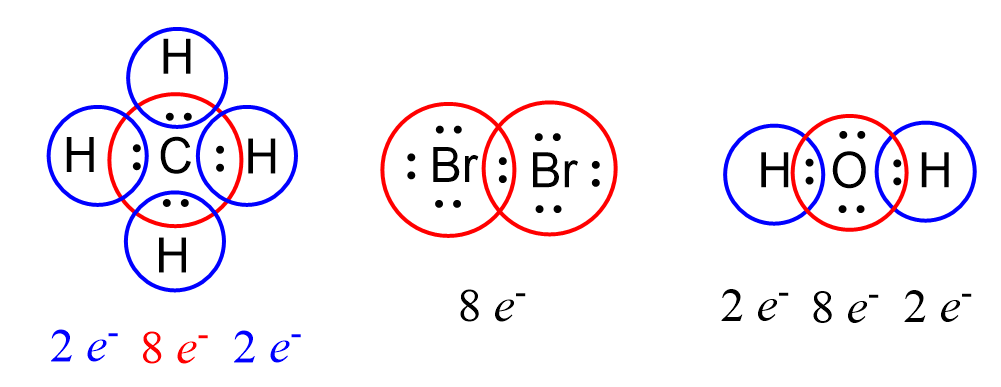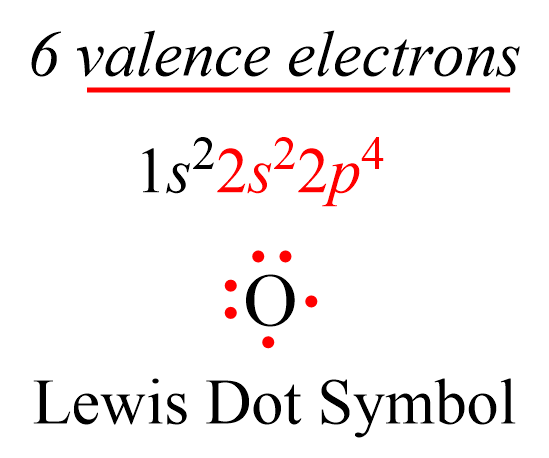Sigma and Pi Bonds
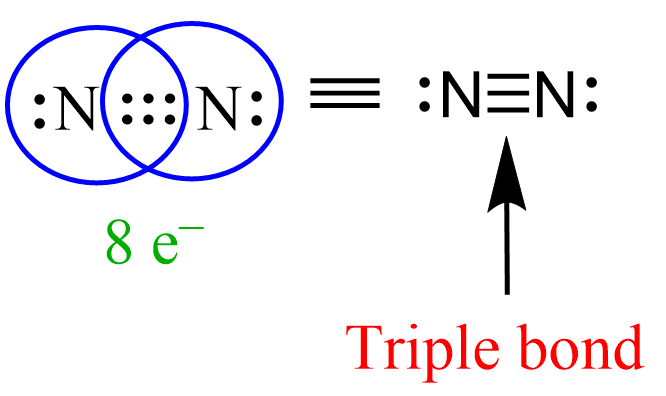
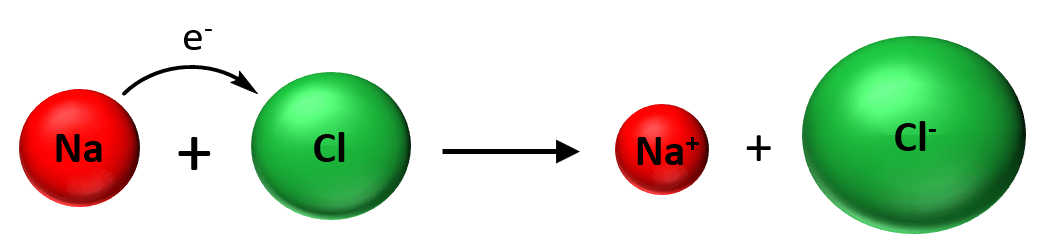
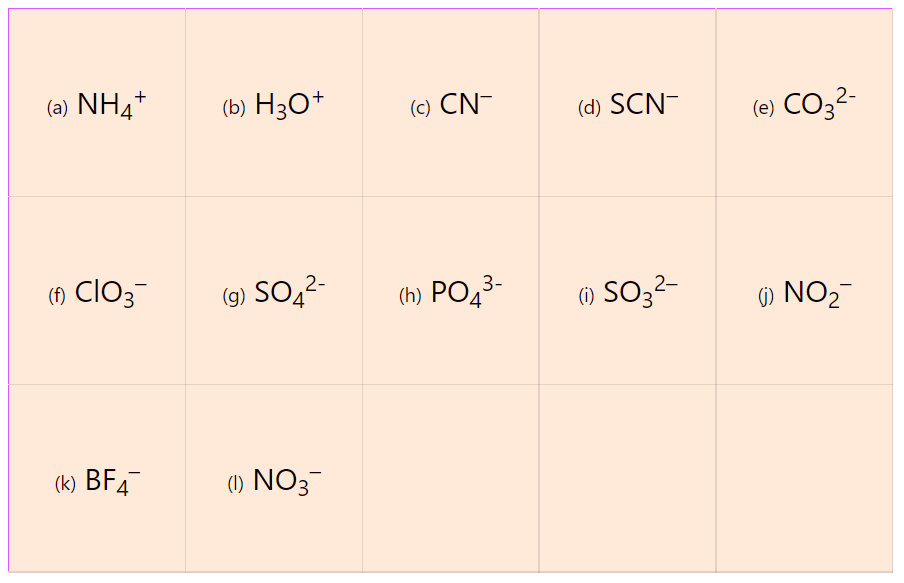
We mentioned in the previous post that covalent bonds are formed as a result of sharing two valence electrons in overlapping orbitals of two atoms. For example, the following Lewis structures represent covalent bonds together with some lone pairs of … Read more