In the previous post, we talked about pH and its measure for acidity.
Remember, pH is defined as the negative logarithm of proton/hydronium ion concentration:
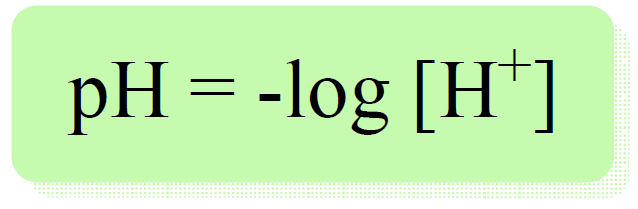
So, how do we know the concentration of proton or hydronium ion from the molarity of an acid?
Well, there are a few possibilities here depending on whether it is a strong or a weak acid. For strong acids such as HCl, HI, HClO4, HNO3, H2SO4 and etc, it is a relatively simple task because, for a strong acid, we assume a 100% dissociation, and therefore, the [H+] is the same as [HA].
Example: Calculate the pH of 2.49 M solution of HNO3.
HNO3 is completely ionized in water producing protons in a 1:1 molar ratio:
HNO3(aq) → H+(aq) +NO3–(aq)
Therefore, [H+] = [HNO3] = 2.49 M, and
pH = -log 2.49 = 0.396
The pH of a Strong Base
It is possible to calculate the pH from the concentration of a base.
For example, what is the pH of 0.450 M solution of NaOH?
The strategy here is to calculate the pOH and then, using the pH + pOH = 14 relationship, determine the pH.
NaOH is a strong base, so [OH–] = [NaOH], and therefore, the pOH is:
pOH = -log 0.450 = 0.346
The pH then is equal to:
pH = 14 – pOH = 14 – 0.346 = 13.7
As expected, the pH is very high because it is a solution of a strong base.
In the next article, we will discuss determining the pH of a weak acid.
Check Also
- Definitions of Acids and Bases
- Acid-Base Reactions
- Acid-Base Titrations
- Conjugate Acid and Conjugate Base
- Autoionization of Water and Kw
- The pH and Acidity
- Acid Strength, Ka, and pKa
- Base Strength, Kb and pKb
- Ka, pKa, Kb, and pKb Relationship
- pH + pOH = 14
- The pH of a Weak Acid
- The pH of a Weak Base
- ThepH of Polyprotic Acids
- The acidity of a Salt Solution
- The pH of a Salt Solution
- The pH of Salts With Acidic Cations and Basic Anions
- Acids and Bases Practice Problems
