To determine if CS2 (carbon disulfide) is polar or nonpolar, we need to first determine its geometry. This presumes knowing the rules for drawing a correct Lewis structure and you can find more details about Lewis structures here.
Check the previous post where we discussed the geometry of CS2 and determined that it is liner:
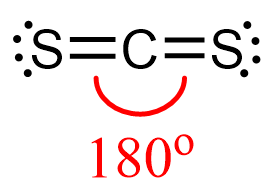
The steric number of carbon is two – two atoms, with no lone pairs, and that indicates a linear geometry.
Now, the polarity: The first thing here is to determine if the C-S bond is polar. Depending on the difference in the electronegativity values, covalent bonds can be polar and nonpolar.

- If the difference in electronegativity is less than 0.5, the electrons are about equally shared between the two atoms, forming a nonpolar a covalent bond.
- If the difference in electronegativity is between 0.5 and 1.7, we have a polar covalent bond.
- A difference of 1.7 or higher is so large that the electrons are no longer shared, and an ionic bond is formed. Ionic bonds are formed between metals and nonmetals.
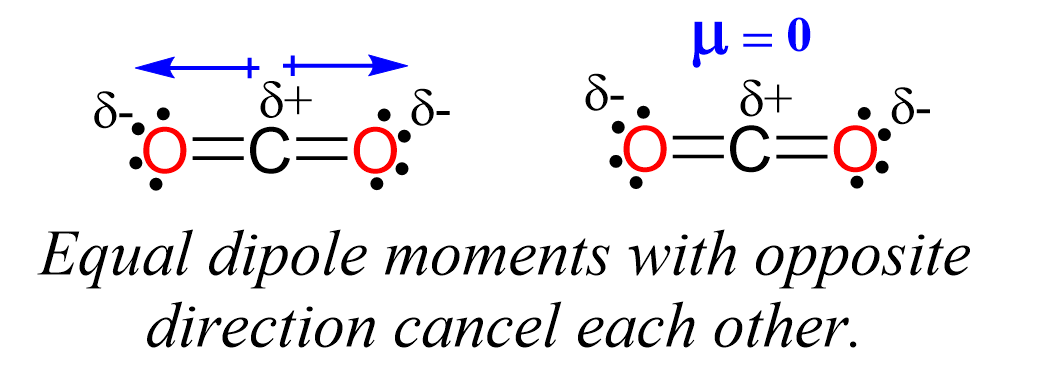
The electronegativities are identical and therefore the C-S bond is nonpolar which means the molecule cannot be polar. Aside from this, notice also that the bonds are at 180o and even if they were polar, the dipoles would’ve been canceled and the molecule would still be nonpolar. Recall the dipoles of C-O bonds in carbon dioxide:
Check this 99-question multiple-choice quiz on Geometry and Hybridization:
Check Also
- The VSEPR Model
- VSEPR Theory Practice Problems
- Hybridization of Atomic Orbitals
- sp, sp2, sp3, sp3d, and sp3d2 Hybridization Practice Problems

