To determine if CO2 is polar or nonpolar, we need to first determine its geometry. This presumes knowing the rules for drawing a correct Lewis structure and you can find more details about Lewis structures here.
Oxygen being more electronegative goes on the periphery:
![]()
For the valence electrons, we have 4 from the carbon and 2 x 6 = 12 from two oxygens, giving 16 valence electrons in total:

One bond between the carbon and each oxygen takes 4 electrons and we have 12 electrons left:

Place 6 on each oxygen and check for the octets:

The oxygens have 8 electrons. However, the carbon has only four and in these cases, you need to move one of the lone pairs, from the element that has an octet to the element lacking an octet to make another bond:

The same is done for the other carbon since it still lacks an octet:

At this point, all the atoms have 8 electrons. Notice that there are four electrons between the carbon and each oxygen which means they make two double bonds:

The steric number of carbon is two – two atoms, with no lone pairs, and therefore, the geometry is linear.
Now, the polarity: The first thing here is to determine if the C-O bond is polar. Depending on the difference in the electronegativity values, covalent bonds can be polar and nonpolar.

- If the difference in electronegativity is less than 0.5, the electrons are about equally shared between the two atoms, forming a nonpolar a covalent bond.
- If the difference in electronegativity is between 0.5 and 1.7, we have a polar covalent bond.
- A difference of 1.7 or higher is so large that the electrons are no longer shared, and an ionic bond is formed. Ionic bonds are formed between metals and nonmetals.
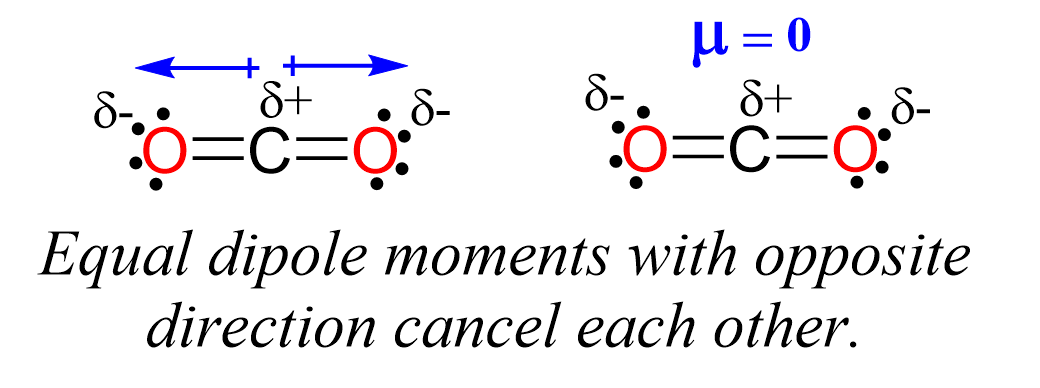
Even though the C-O bond is polar, the dipole moments of C-O bonds are canceled because they point in opposite directions, and we conclude that CO2 is a nonpolar molecule.
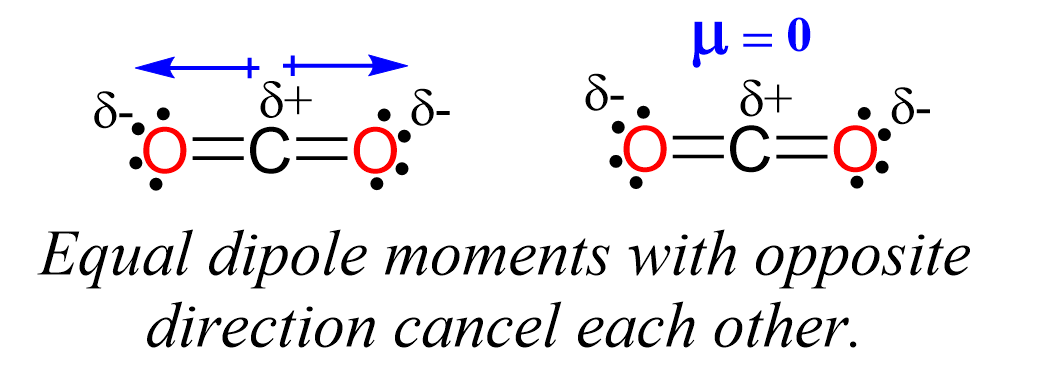
Remember, the net dipole of the molecule is the vector sum of all the dipoles and here it equals zero because the bonds are equivalent and pointing in opposite directions.
We have also discussed the hybridization of CO2 in this article.
Check this 99-question multiple-choice quiz on Geometry and Hybridization:
Check Also