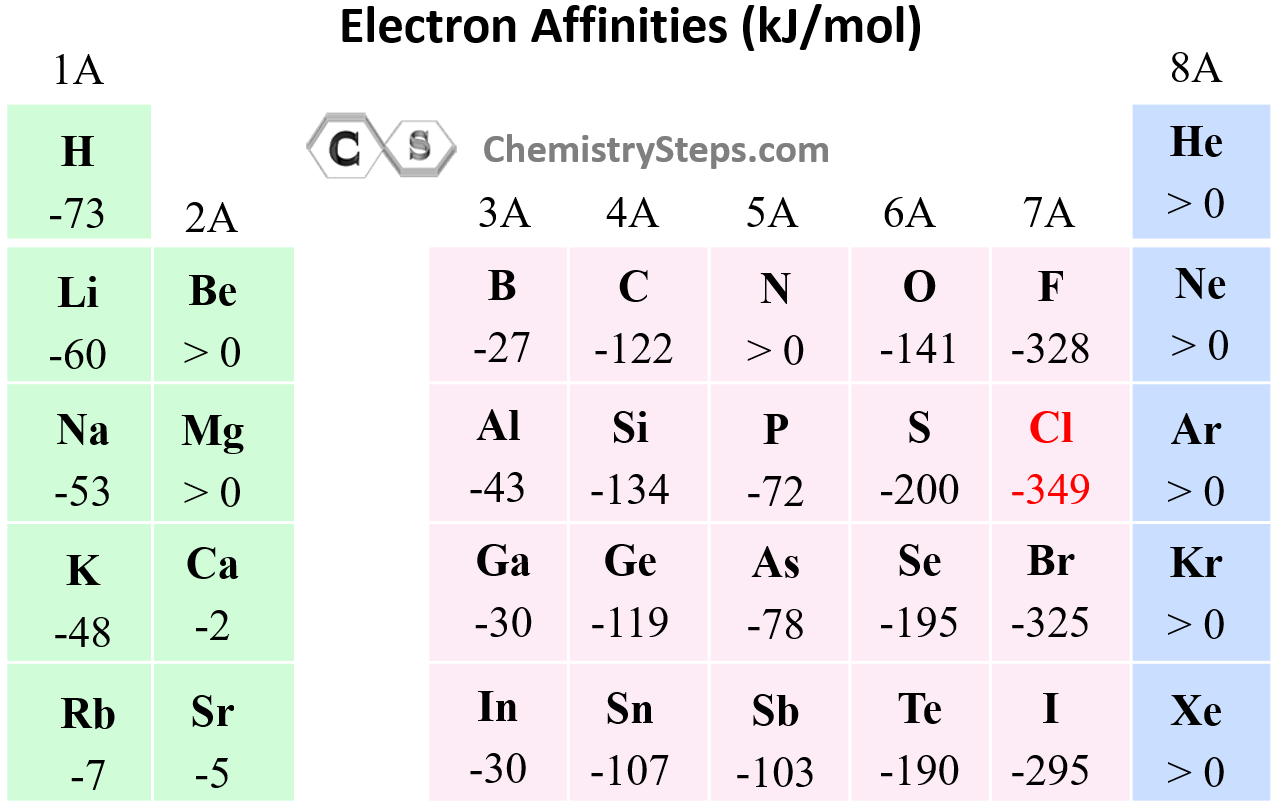
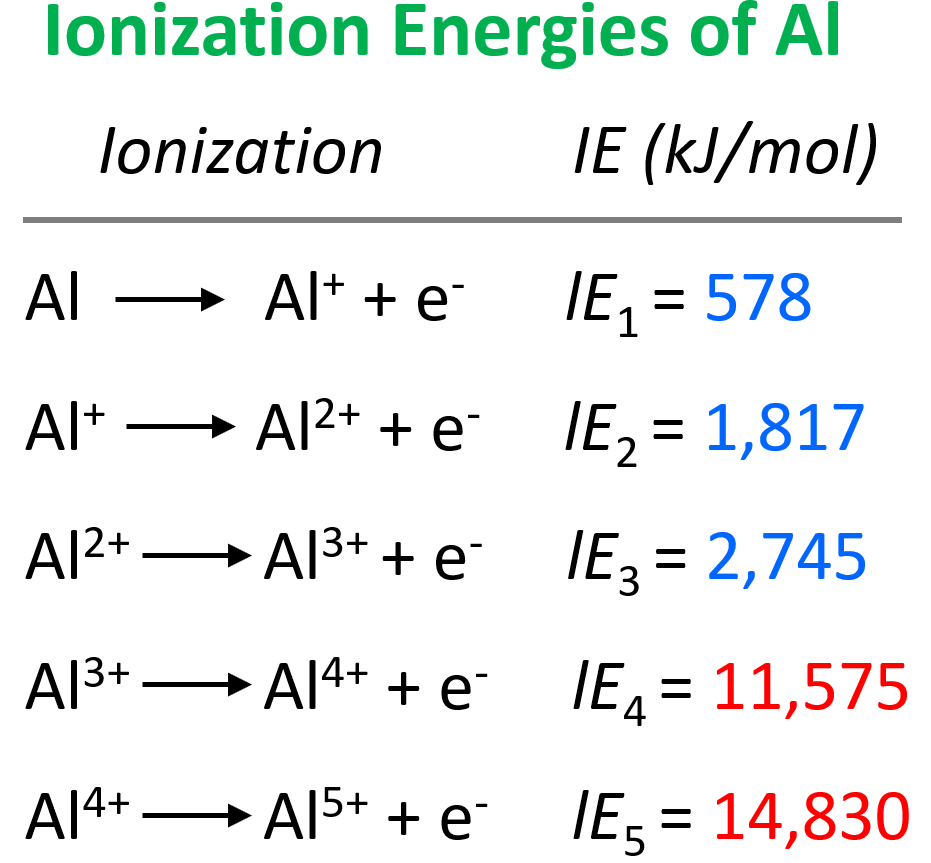
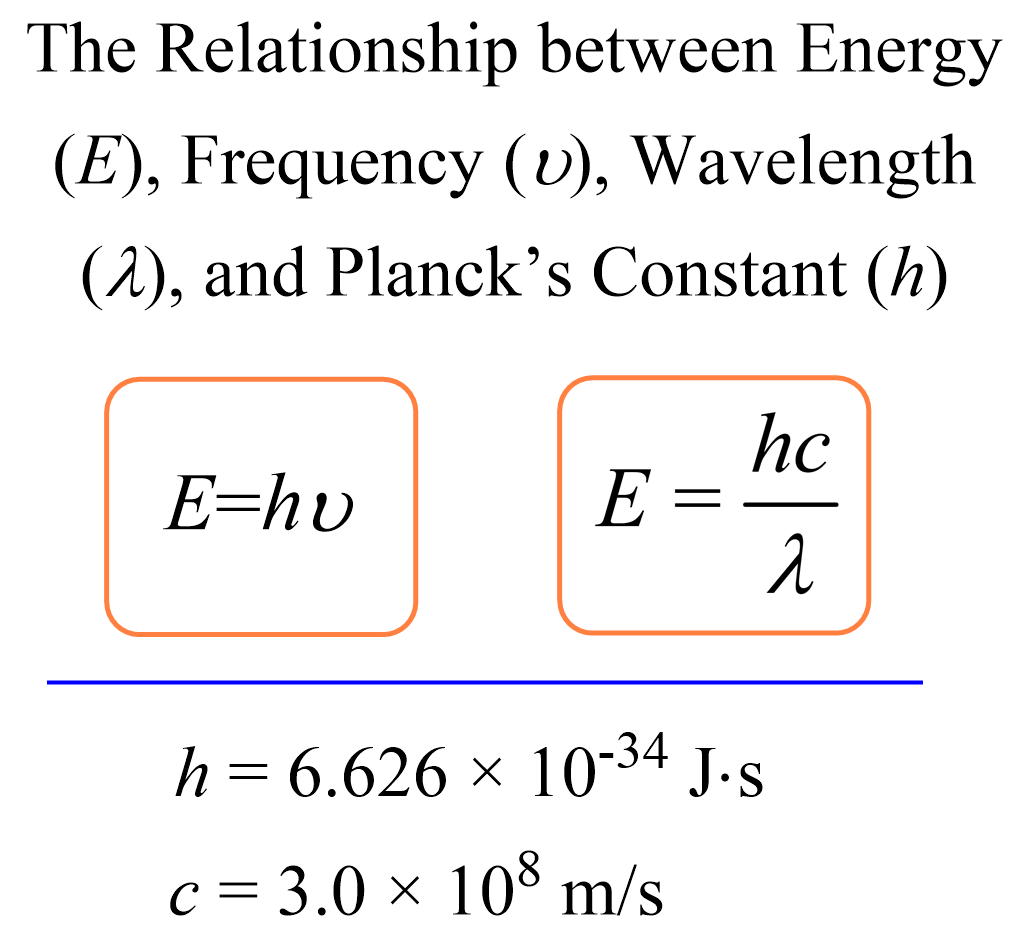
Electron Affinity
In the previous post, we talked about ionization energy which is the amount of energy required to remove an electron from the isolated neutral atom in the gaseous state. During the ionization, a neutral atom was becoming a … Read more