To determine if H2O (water) is polar or nonpolar, we need to first determine its geometry. This presumes knowing the rules for drawing a correct Lewis structure and you can find more details about Lewis structures here.
There are 6 + 2 = 8 electrons in the molecule, and four are used for making the two O-H bonds, and four are on the oxygen as lone pairs:
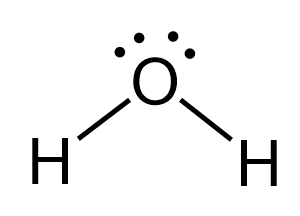
The central atom has a steric number of 4 – two atoms and two lone pairs, and therefore, the electron geometry, therefore, is tetrahedral, and the molecular geometry is bent.
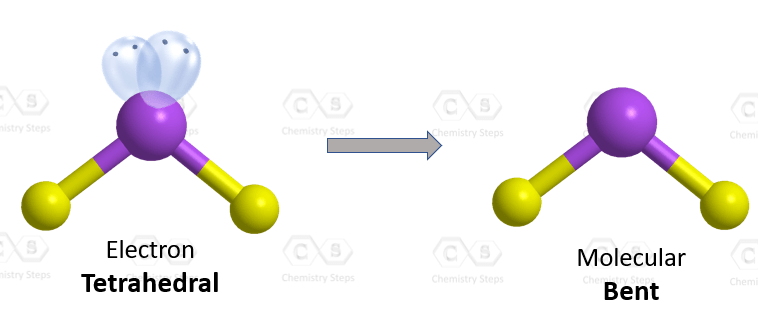
As expected, the two lone pairs make the bond angle between the hydrogen atoms even smaller bringing it down to 104.5o.
Now, the polarity: The first thing here is to determine if the O-H bond is polar. Depending on the difference in the electronegativity values, covalent bonds can be polar and nonpolar.

- If the difference in electronegativity is less than 0.5, the electrons are about equally shared between the two atoms, forming a nonpolar a covalent bond.
- If the difference in electronegativity is between 0.5 and 1.7, we have a polar covalent bond.
- A difference of 1.7 or higher is so large that the electrons are no longer shared, and an ionic bond is formed. Ionic bonds are formed between metals and nonmetals.
Oxygen is more electronegative and therefore, the dipole moment of the OH bonds is towards oxygen and the overall dipole is pointing up as shown below.
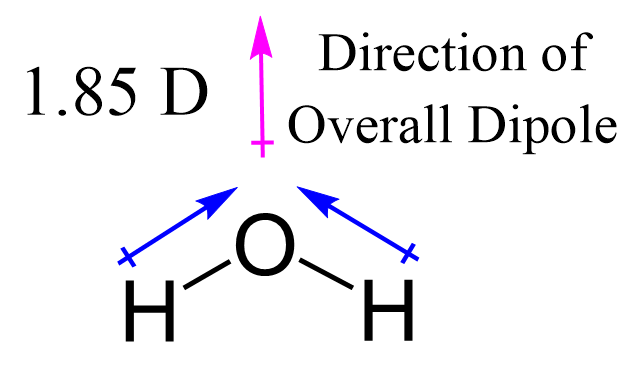
Water is the most common and important polar molecule. It dissolves polar inorganic molecules such salts, acids, and bases because of its polarity.
Check this 99-question multiple-choice quiz on Geometry and Hybridization:
Check Also

