Percent Composition and Empirical Formula Practice Problems
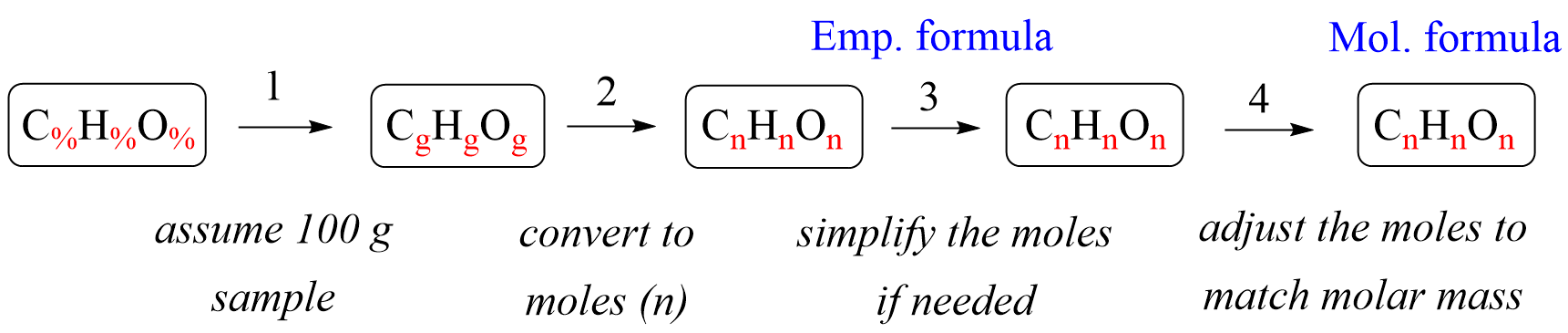
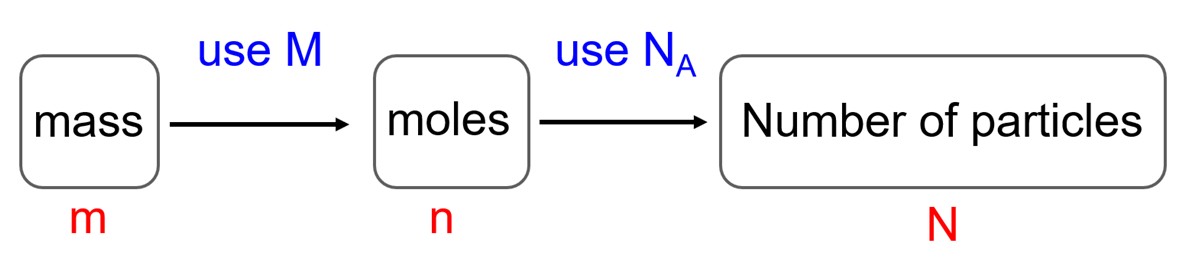
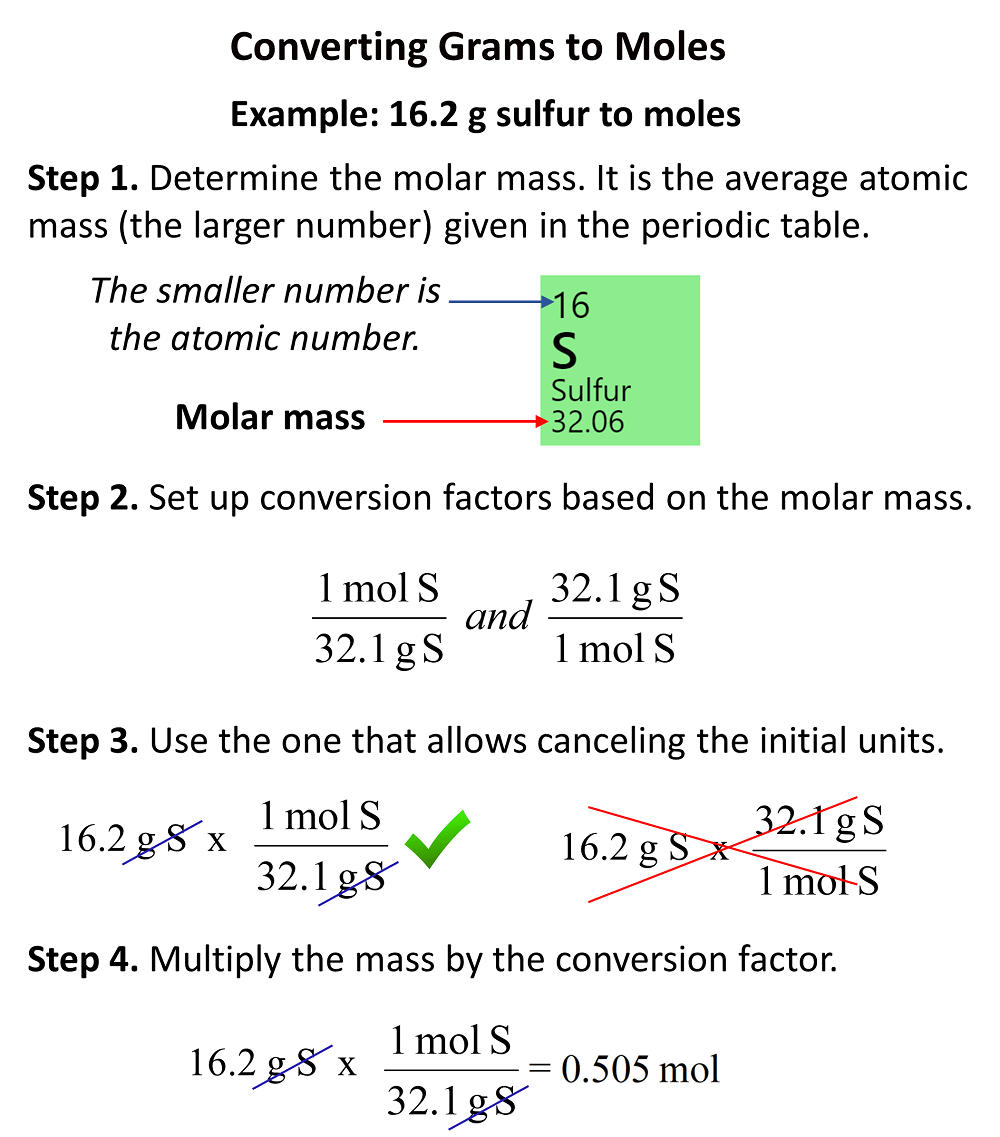
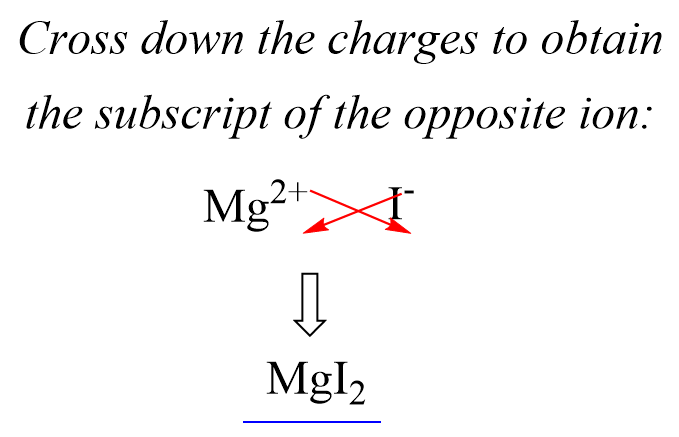
In the previous post, we talked about the percent composition and its use for determining the empirical and molecular formulas of compounds. Remember, the percentage composition shows the amount of each element in a compound expressed as a mass percent … Read more