In this post, we will be drawing the Lewis structure, and determining the geometry and hybridization of atosms in carbon disulfide, CS2.
CS2 Lewis Structure
The first thing we need to do when drawing a Lewis structure is determine the total number of valence electrons in the molecule. Remember, valence electrons are those in the outermost principal energy level. For example: Na – 1s22s22p63s1, Cl – 1s22s22p63s23p5
The number of valence electrons, for main group elements, corresponds to their group number in the periodic table:
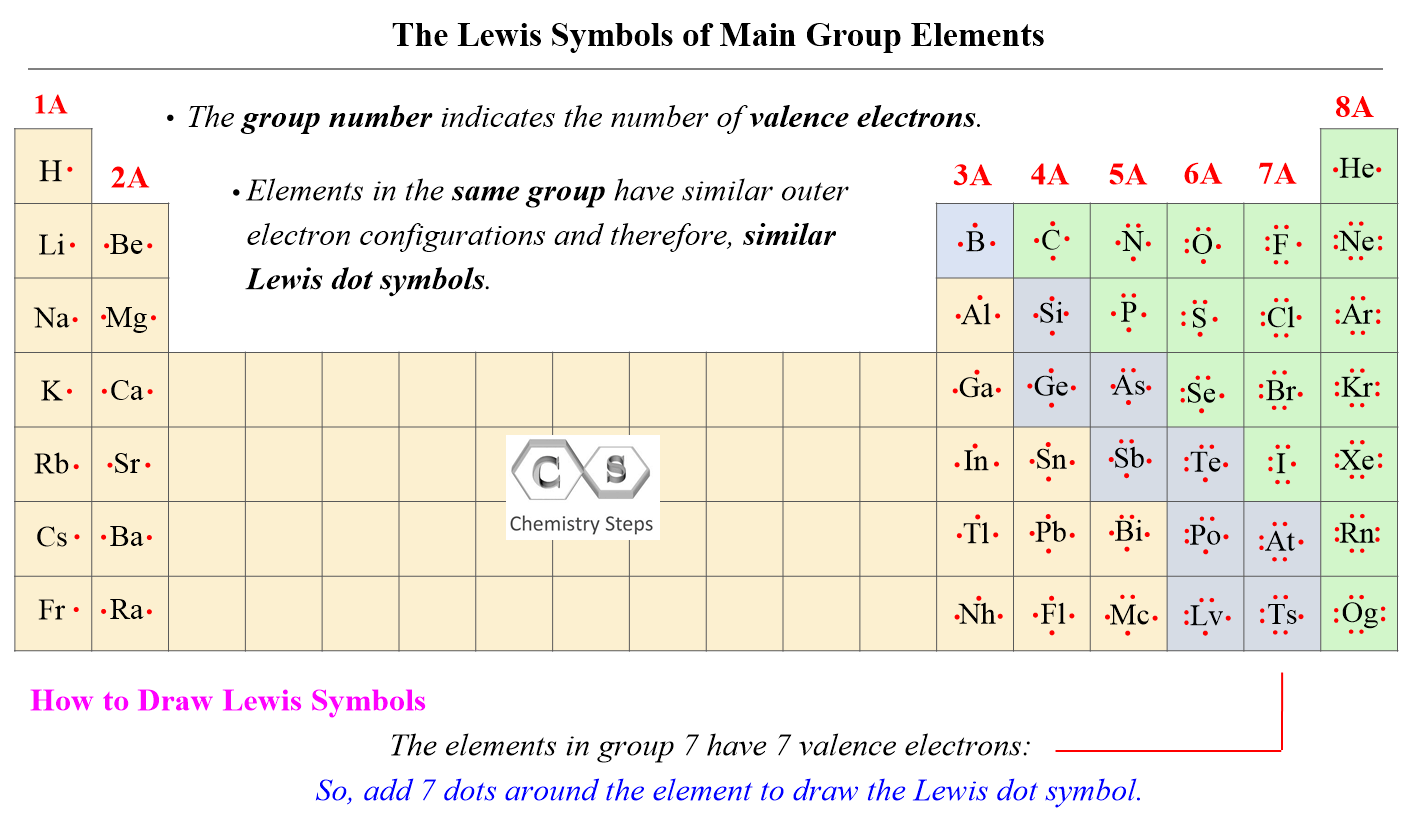
For d block elements, the outermost d electrons are also counted as valence electrons (ns + (n-1)d). For example, iron has eight valence electrons: Fe – 1s22s22p63s23p64s23d6.
So, carbon is in group 4A and sulfur is in group 6A, and therefore, they have four and six valence electrons respectively which makes 4 + 2 x 6 = 16 valence electrons in total.
Next, we need to connect the atoms in the correct order and add the electrons as bonds and lone pairs.
In short, these are the steps you need to follow for drawing a Lewis structure:
1. Write the correct skeletal structure for the molecule.
* Hydrogen atoms are always terminal (only one bond)
* Put more electronegative elements in terminal positions
2. Sum the valence electrons from all the atoms.
3. Use a pair of electrons to form a bond between each pair of bound atoms.
4. Add the remaining electrons to satisfy the octet for a more electronegative atom first.
5. If any atoms lack an octet, make a double or triple bond to give them an octet.
Out of the 16 electrons, four are used to make two bonds between the three atoms:
![]()
Notice that the bonds can be shown as a line and in fact, this is the most common way of showing structures once we learn the principles of Lewis structures.
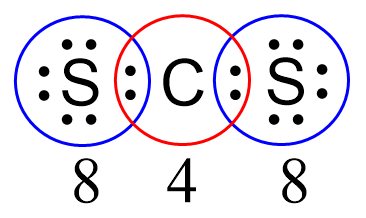
There are 12 electrons left which go to the sulfur atoms as three lone pairs on each:
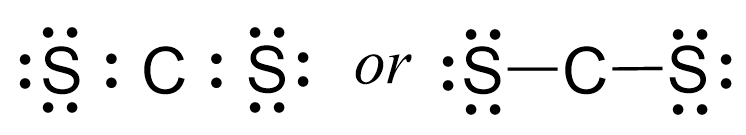
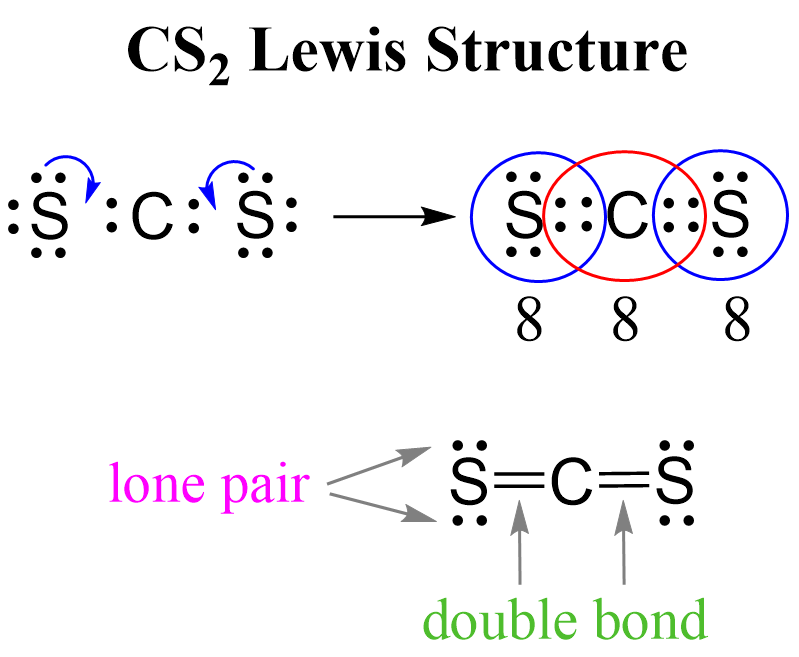
Next, check if the atoms have octets. Each sulfur is surrounded by eight electrons, however, carbon has only four:
What we do to make an octet is move one of each lone pairs to make a double bond:
CS2 Hybridization
The steric number of carbon is two – two atoms, with no lone pairs, and therefore, the hybridization is sp.
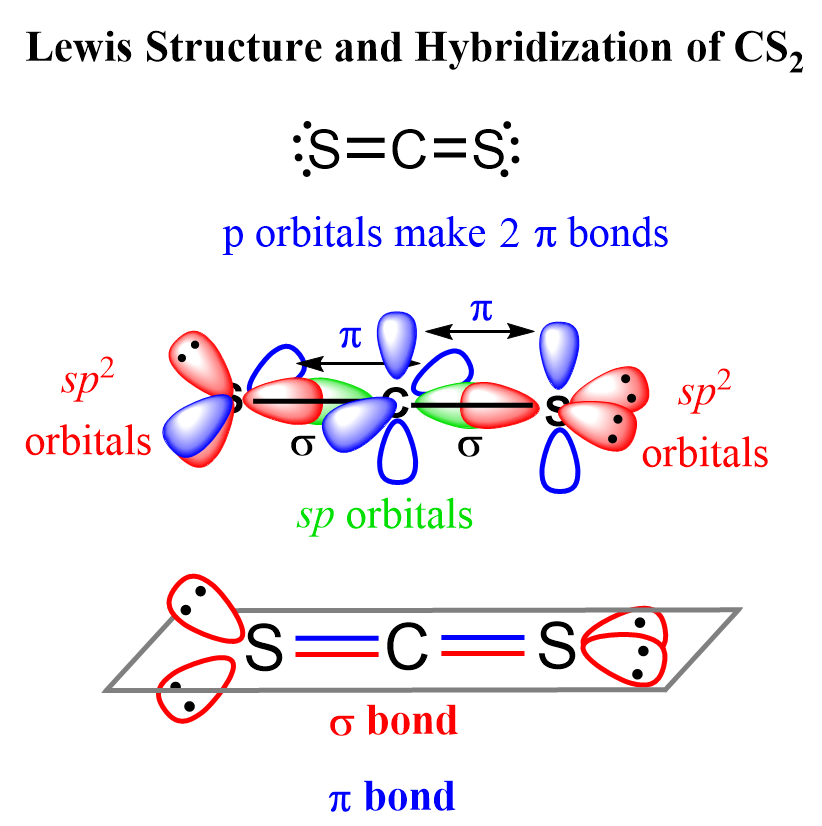
The sigma bond with each sulfur is formed by the linear overlap of the C(sp) and S(sp2) orbitals, while the pi bonds are formed by a side-to-side orbital overlap of the unhybridized p orbitals of the carbon and sulfur.
An interesting feature about this geometry is that the sp2 orbitals, containing the lone pairs of the sulfur, are at 90o because the unhybridized p orbitals of carbon are also oriented 90° to each other, and in order to overlap and make the pi bonds, the p orbitals must be parallel.
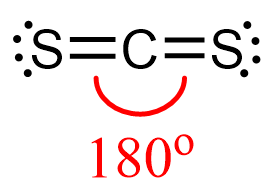
CS2 Geometry
Steric number two corresponds to linear geometry with bond angles of 180o.
Check this 99-question multiple-choice quiz on Geometry and Hybridization:
Geometry and Hybridization Quiz
Check Also
- Lewis Dot Symbols
- Valence Electrons
- The VSEPR Model
- Lewis Structures Practice Problems
- VSEPR Theory Practice Problems
- Hybridization of Atomic Orbitals
- sp, sp2, sp3, sp3d, and sp3d2 Hybridization Practice Problems

