First, we need to draw the Lewis structure of H2O.
In short, these are the steps you need to follow for drawing a Lewis structure:
1. Write the correct skeletal structure for the molecule.
* Hydrogen atoms are always terminal (only one bond)
* Put more electronegative elements in terminal positions
2. Sum the valence electrons from all the atoms.
3. Use a pair of electrons to form a bond between each pair of bound atoms.
4. Add the remaining electrons to satisfy the octet for a more electronegative atom first.
5. If any atoms lack an octet, make a double or triple bond to give them an octet.
Oxygen being more electronegative goes on the periphery and there are 6 + 2 = 8 electrons, four out of which are used to make the two bonds, and the other four go to the oxygen as lone pairs:

The central atom has a steric number of 4 – two atoms and two lone pairs. The electron geometry, therefore, is tetrahedral, and the molecular geometry is bent.
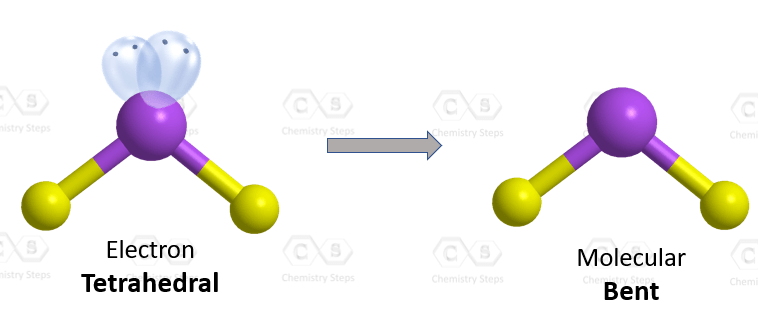
The atoms at the lone pair are expected to be at 109.5o, however, because the revulsion from the lone pair is stronger, the angle between the hydrogens is about 104.5o:

Strict number four corresponds to sp3 hybridization.
Check this 99-question multiple-choice quiz on Geometry and Hybridization:
Check Also

