In any oxidation-reduction reaction, there is an oxidizing agent and a reducing agent as oxidation cannot occur with reduction and vice versa, reduction cannot occur without oxidation. The oxidizing agent takes electron(s) from the reducing agent, so we can also say that the reducing agent gives electron(s) to the oxidizing agent.
For example, oxygen is naturally the most common oxidizing agent which, among others, can oxidize metals to their oxides. In fact, historically, the term oxidation refers to the combination of an element with oxygen to yield an oxide. This is a good reference for assessing the change in oxidation numbers to classify the reactant as an oxidizing or reducing agent.
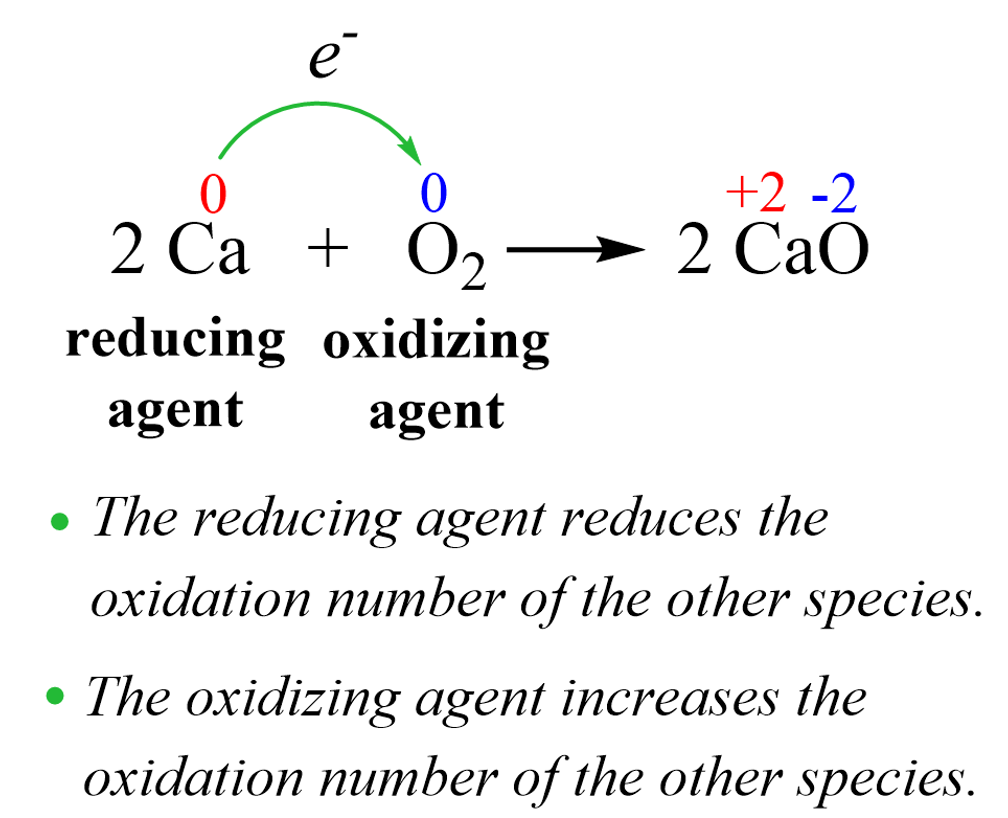
Oxygen, being the oxidizing agent, takes the electrons from calcium thus increasing its oxidation number to +2. So, as a general description, we can say that the oxidizing agent is a substance that causes the oxidation of another substance. The reducing agent, then, is the one that reduces another substance thus decreasing its oxidation number.
The Correlation Between Oxidizing/Reducing Agents and Species being Reduced or Oxidized
This may be confusing, but you need to remember that:
- The oxidizing agent is being reduced.
- The reducing agent is being oxidized.
There is quite a lot of terminology in redox reactions, and it can get confusing. There are different ways of memorizing them.
For example, going with oil rig:
Oxidation is a loss (of electrons)
Reduction is a gain (of electrons)
I’d recommend going with oxygen. In most reactions, it is an oxidizing agent and goes from 0 to -2. So, you can go based on the fact that the oxidation number of the oxidizing agent decreases. The most common oxidation reaction is probably the combustion reaction where O2 is the oxidizing agent.
For example, identify the oxidizing and reducing agent, the element that is oxidized, and the element that is reduced in the following redox reaction:
2KMnO4 + 16HCl → 2MnCl2 + 5Cl2 + 2KCl + 8H2O
Remember, not all the atoms undergo oxidation or reduction in a redox reaction. So, to find the species that undergo a redox transformation, look for atoms that are in different forms on both sides of the equation. In this reaction, we see that chlorine is in a free form on the right side of the equation while on the left side, it is a chloride ion. The manganese is also in a different environment, and it would be a good idea to compare its oxidation states.
The hydrogen and oxygen are almost always in their standard oxidation state unless they are in free forms. The exceptions of hydrogen are also when it is bound to a metal, while oxygen deviates from the standard -2 when connected to fluorine or to itself like in hydrogen peroxide. These exceptions are mentioned in the rules for determining the oxidation numbers:
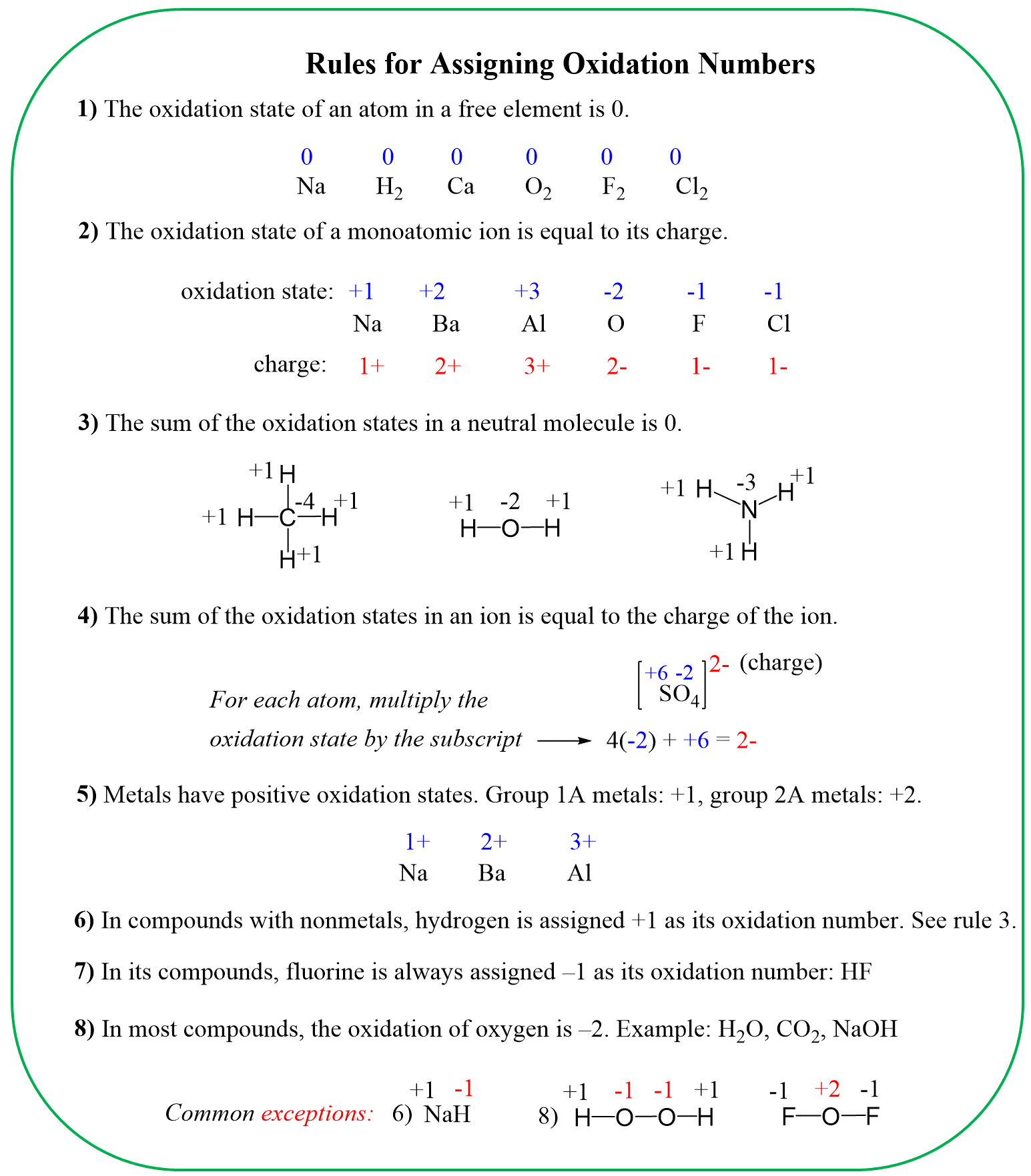
So, the candidate molecules are KMnO4, MnCl2, HCl, and Cl2.

Potassium permanganate is the oxidizing agent because Mn is in high oxidation state (+7) and it oxidizes the chlorine in HCl to Cl2. The oxidation number of chlorine changes from -1 to 0. Chlorine gives electrons to Mn and therefore, HCl is the reducing agent.
Another example: determine the oxidizing and reducing agent in the following reaction:
Fe2+(aq) + Cr2O72-(aq) → Fe3+(aq) + Cr3+(aq)
The oxidation number of iron in Fe2+ is +2 and it increases to +3, so it is being oxidized by the dichromate ion, Cr2O72-. So, Cr2O72- is the oxidizing agent. The oxidation state of chromium in Cr2O72- is +6, and it becomes +3 in Cr3+. It gains electrons from Fe2+, and therefore is being reduced by Fe2+ which is the reducing agent.
Let’s confirm this by setting up an equation to determine the oxidation number of chromium.
Rule 8 – The oxidation of oxygen is –2:
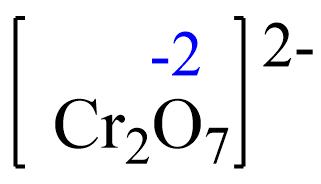
Assign x for the oxidation state of Cr and set up an equation. Remember, for ions, the sum of the oxidation numbers multiplied by their subscripts must be equal to the charge of the ion:
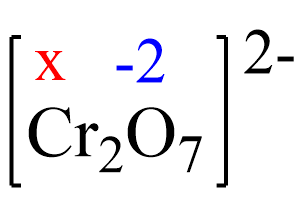
2x + 7(-2) = -2, x = +6
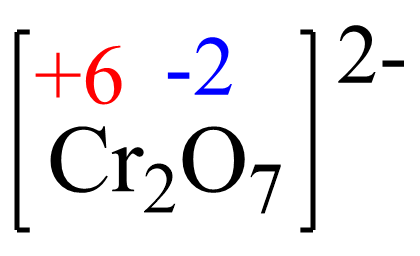
Another example: Determine the oxidizing agent in the following redox reaction. What element is being oxidized?
PbO2(s) + Mn2+(aq) + SO42–(aq) → PbSO4(s) + MnO4–(aq)
The atom that accepts an electron(s) is said to be reduced, and the one that gives the electron(s) is said to be oxidized.
Oxidation is the process of losing an electron(s), and reduction is the process of gaining an electron(s).
- The oxidizing agent is being reduced.
- The reducing agent is being oxidized.
The first thing is to determine the oxidation numbers.
For MnO4– the element with a standard oxidation state is oxygen which is -2, and we need to determine the oxidation state of the manganese.
Let’s confirm this by setting up an equation.
Rule 8 – The oxidation of oxygen is -2:
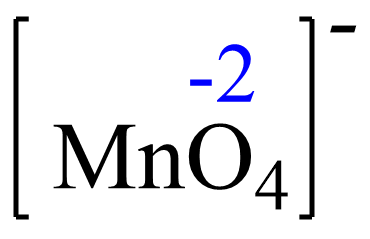
Assign x for the oxidation state of Mn and set up an equation:
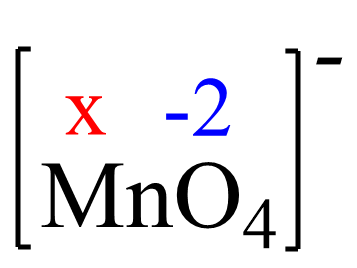
x + 4(-2) = -1, x = +7
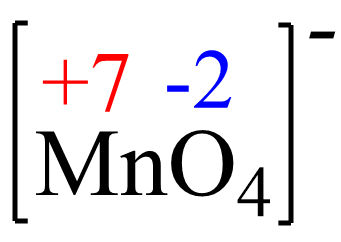
The oxidation state of Mn in MnO4– is +7, so it is increasing from +2 to +7 and thus being oxidized. So. MnO4- is the reducing agent here and this is a rare reaction where you will see Mn2+ being oxidized to MnO4– as, most often, it is the permanganate ion that is an oxidizing agent, in it converts to Mn2+.
We can then conclude that PbO2 is the oxidizing agent. The oxidation state of Pb in Pb2+ is +2 according to rule 2. In PbO2, it is +4, so the oxidation state of Pb decreases from +4 to +2, and therefore, it is being reduced.
One more example where a common oxidizing agent hydrogen peroxide acts as a reducing agent because the other reactant is even a stronger oxidizing agent: What element is being reduced in the following redox reaction?
H2O2(aq) + ClO2(aq) → ClO2–(aq) + O2(g)
The atom that accepts an electron(s) is said to be reduced, and the one that gives the electron(s) is said to be oxidized.
Oxidation is the process of losing an electron(s), and reduction is the process of gaining an electron(s).
- The oxidizing agent is being reduced.
- The reducing agent is being oxidized.
Let’s determine the oxidation states starting with H2O2.
Rule 6 – The oxidation of hydrogen is +1:
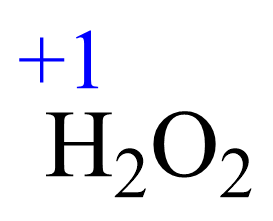
Assign x for the oxidation state of O and set up an equation:
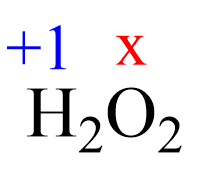
2(+1) + 2x = 0, x = -1
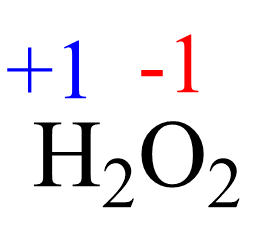
This is an unusual oxidation number of oxygen in a molecule, and the reason is that the two oxygens are connected, so they each pull the electron density only from one hydrogen atom:
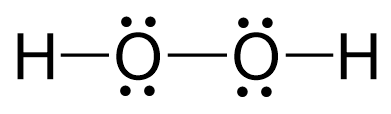
We discussed the Lewis structure and hybridization of H2O2 in a separate article which you can find here.
For ClO2:
Rule 8 – The oxidation of oxygen is -2:
Assign x for the oxidation state of C and set up an equation:
x + 2(-2) = 0, x = +4
The oxidation state of oxygen in O2 is 0, so let’s determine the oxidation state of Cl in ClO2–.
Rule 8 – The oxidation of oxygen is –2:
Assign x for the oxidation state of C and set up an equation:
x + 2(-2) = -1, x = +3
The oxidation state of chlorine decreases from +4 to +3. It gains electrons and therefore is being reduced.
More examples of the oxidation state in this multiple-choice quiz:
Oxidation State and Redox Reactions Quiz
Reactions in Aqueous Solutions Quiz
Check Also
- Solutions
- Strong and Weak Electrolytes
- Dissociation of Ionic Compounds
- Molecular, Ionic, and Net Ionic Equations
- Molarity
- Dilution
- Ion Concentration
- Precipitation Reactions
- Definitions of Acids and Bases
- Acid-Base Reactions
- Stoichiometry of Reactions in Aqueous Solutions
- Acid-Base Titrations
- Oxidation-Reduction (Redox) Reactions
- Reactions in Aqueous Solutions Practice Problems

