In a precipitation reaction, an insoluble product is formed which is called a precipitate. Precipitation reactions usually involve ionic compounds. For example, when an aqueous solution of sodium chromate (Na2CrO4) is added to an aqueous solution of silver nitrate (AgNO3), a dark orange precipitate of silver chromate (Ag2CrO4) is formed:
Na2CrO4(aq) + 2AgNO3(aq) → Ag2CrO4(s) + 2NaNO3(aq)

Predicting the Products of a Reaction
If we compare the formulas of the reactants and products in the previous reaction, we can see that an exchange of ions had occurred; the cation of one salt combined with the anion of the other salt and vice versa. These types of reactions are called metathesis or double-displacement reactions.
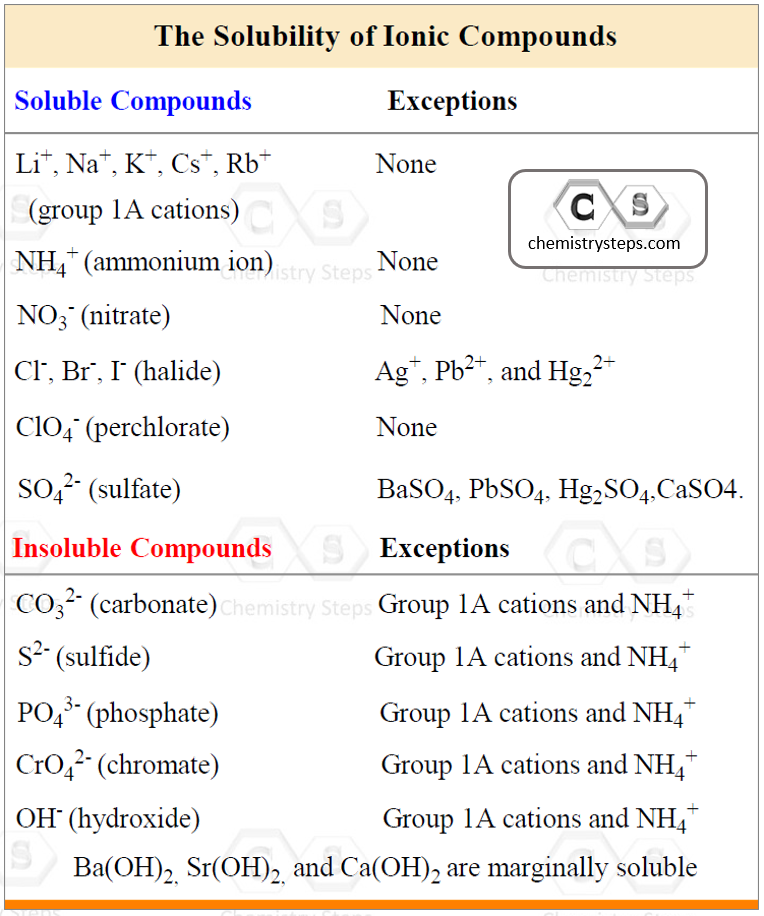
To be able to predict the products of a double displacement reaction, you need to know the formulas and the charges of the ions in the starting materials and use the solubility rules to identify the precipitate in the reaction.
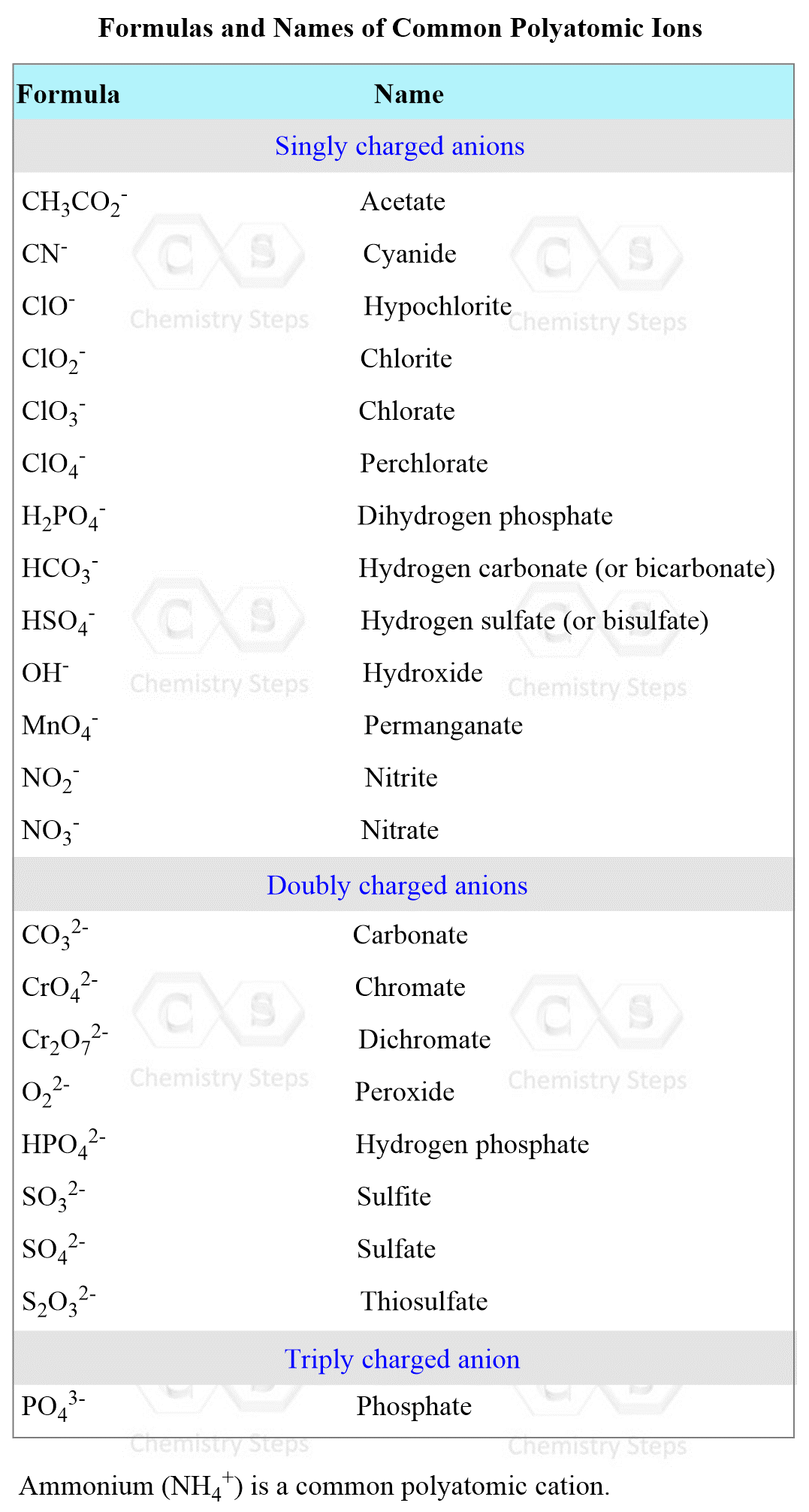
Let’s use the table of the most common polyatomic ions, and determine the products of the reaction between AgNO3 and CaCl2:
Complete and balance the following equation between solver nitrate and calcium chloride:
AgNO3(aq) + CaCl2(aq) → ?
1) Exchange the cations and anions. Only carry the subscripts that are part of the formula. I.e., we don’t carry the subscript of Cl, but we do for the nitrate since the formulas are Cl– and NO3–:
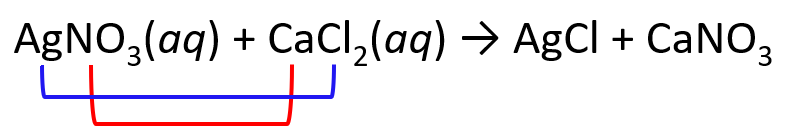
2) Place the ionic charges on top of elements and adjust the subscripts:
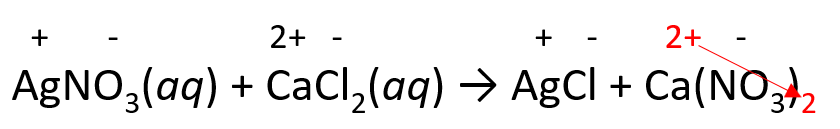
3) Balance the equation with coefficients and remove the charges:
2AgNO3(aq) + CaCl2(aq) → 2AgCl + Ca(NO3)2
4) Label the soluble compounds as (aq). Determine the precipitate (in this case AgCl) and label it as (s) – solid.
2AgNO3(aq) + CaCl2(aq) → 2AgCl(s) + Ca(NO3)2(aq)
AgCl is a solid (precipitate) because the salts of halides are soluble except when combined with Ag+, Pb2+, and Hg22+.
Being able to predict the products of a reaction is very important partly because it is required for solving problems on determining the amount of the product based on the stoichiometry of the reaction.
In the next post, we will talk about the stoichiometry of reactions in aqueous solutions and examples of precipitation and acid-base reactions.
Check Also
- Solutions
- Strong and Weak Electrolytes
- Dissociation of Ionic Compounds
- Molecular, Ionic, and Net Ionic Equations
- Molarity
- Dilution
- Ion Concentration
- Definitions of Acids and Bases
- Acid-Base Reactions
- Stoichiometry of Reactions in Aqueous Solutions
- Acid-Base Titrations
- Oxidation State
- Oxidation-Reduction (Redox) Reactions
- Reactions in Aqueous Solutions Practice Problems
Practice
Complete and balance each of the following molecular equations in aqueous solution:
a) AgNO3(aq) + CaCl2(aq) →
b) HNO3(aq) + Na2CO3(aq) →
c) K2CO3(s) + HClO3(aq) →
d) Al(OH)3(s) + HBr(aq) →
e) H3PO4(aq) + Fe(OH)3(s) →
f) BaCl2(aq) + K2SO4(aq) →

Thanks for the great work you do