Before talking about electrolytes, let’s formulate the concept of solutions. A solution is a homogeneous mixture of two or more substances. The substance present in the greatest quantity is called the solvent, and the other substance(s) is called solute(s). The solute is dissolved in the solvent.
An aqueous solution is one in which water acts as the solvent. This is the type of solution that you will be working in the entire course of general chemistry.
For example, dissolving a pill in a glass of water makes an aqueous solution of the components in the pill:

The mass of the solution is equal to the sum of the masses of the solvent and the solute.
For example, if we dissolve 2 grams of table salt in 98 g of water, we will obtain a 100 g aqueous solution. In this solution, water is the solvent, and the salt is the solute.
Although water is going to be the solvent for most of the solutions, it can also be a solute when mixed with a liquid in greater quantity.
For example, rubbing alcohol can be a mixture of 70% isopropanol (solvent) and 30% water (solute).

How Compounds dissolve in Water
The way a compound dissolves in water depends on the type of its bonding. Ionic compounds such as acids, bases, and salts dissociate in water forming ions that are surrounded by water molecules. Water molecules are the driving force for this separation and caging of ions which is called solvation.
For example, sodium chloride (NaCl) dissociates into Na+ and Cl– ions when dissolved in water:
NaCl(aq) → Na+(aq) + Cl–(aq)
The “aq” notation indicates that the salt is water-soluble, and the ions are surrounded by water molecules.
Molecular compounds such as sucrose or ethanol where the atoms are connected with covalent bonds, do not dissociate into ions. The molecules are dispersed throughout the solution without breaking into new particles.
Electrolytes Nonelectrolytes
All solutes that dissolve in water are classified as electrolytes or nonelectrolytes. Electrolytes are substances that dissolve in water to form solutions that conduct electricity.
The aqueous solutions of nonelectrolytes, on the other hand, do not conduct electricity.
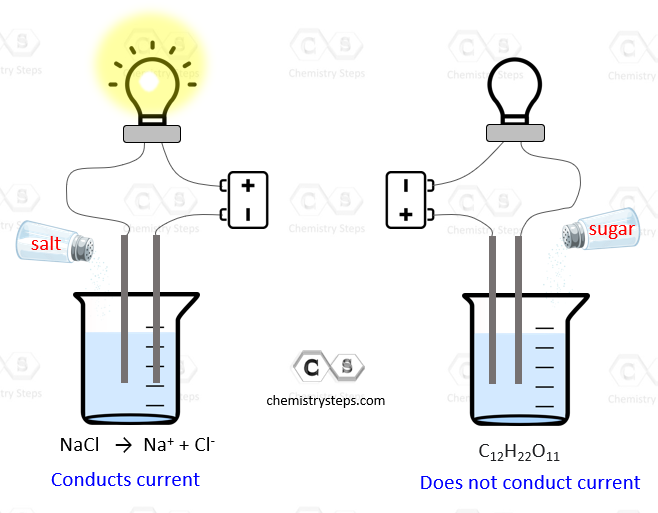
The basis of this feature of electrolytes is their dissociation into ions when dissolved in water. The ions are charged particles that support the electric current while nonelectrolytes cannot serve as a link closing the electrical circuit.
There is a separate post about electrolytes and nonelectrolytes where we will cover the key principles of writing dissociation equations.
Check Also
- Strong and Weak Electrolytes
- Dissociation of Ionic Compounds
- Molecular, Ionic, and Net Ionic Equations
- Molarity
- Dilution
- Ion Concentration
- Precipitation Reactions
- Definitions of Acids and Bases
- Acid-Base Reactions
- Stoichiometry of Reactions in Aqueous Solutions
- Acid-Base Titrations
- Oxidation State
- Oxidation-Reduction (Redox) Reactions
