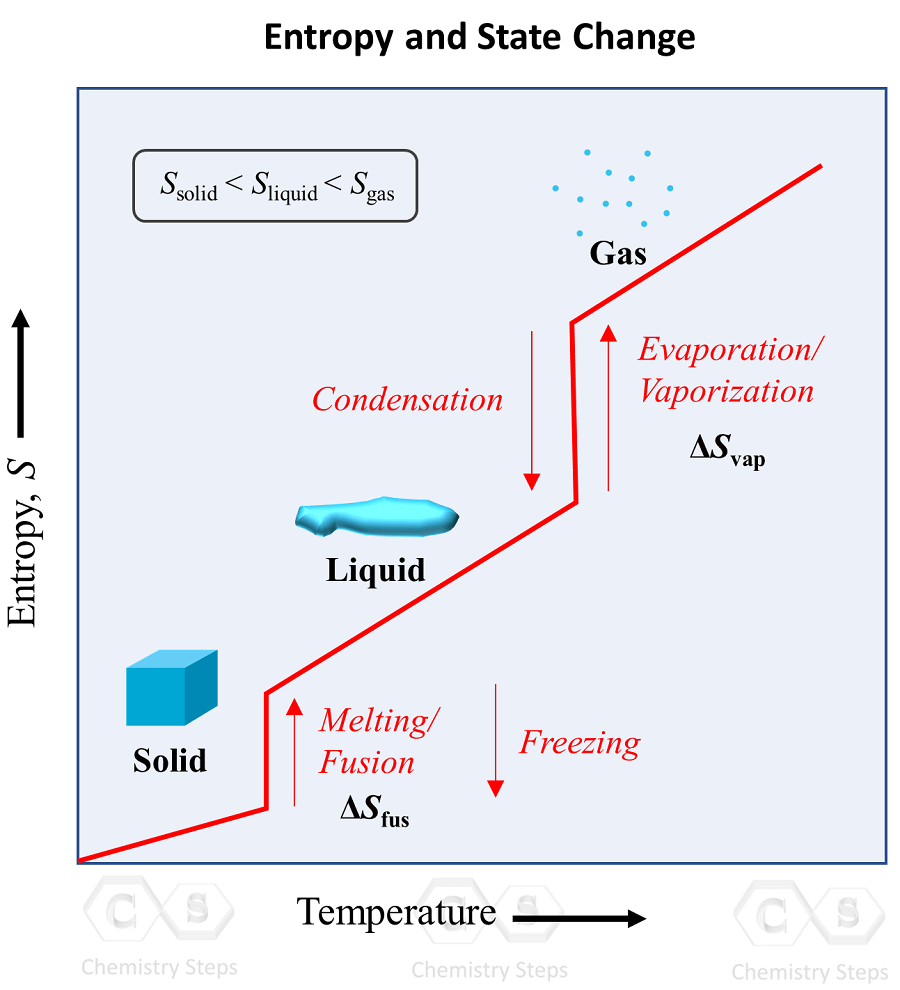
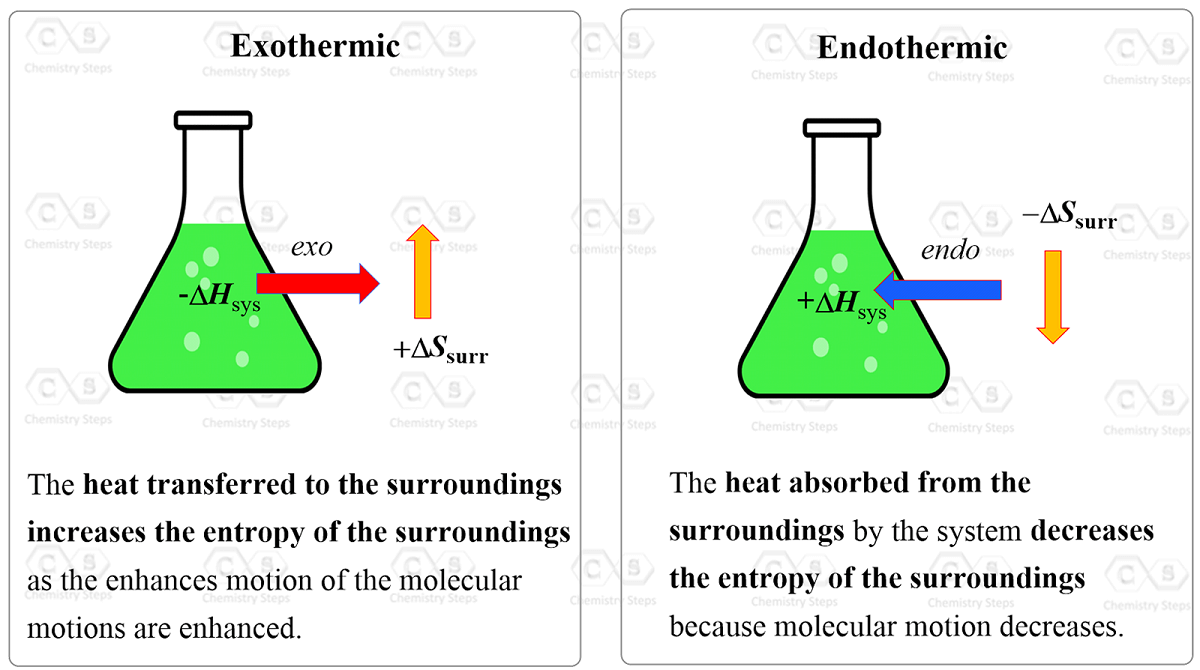
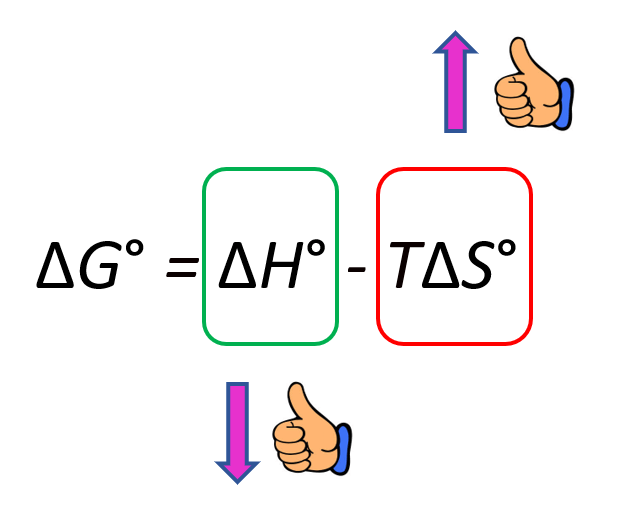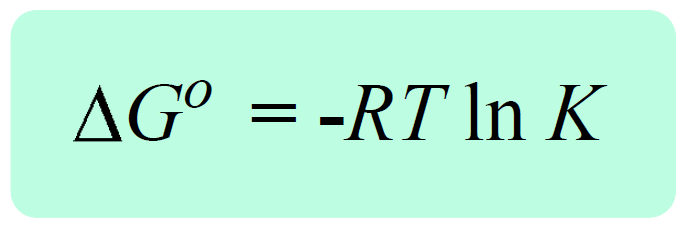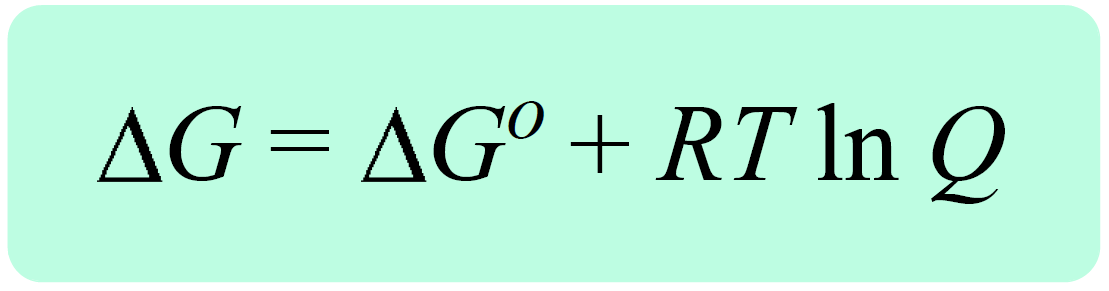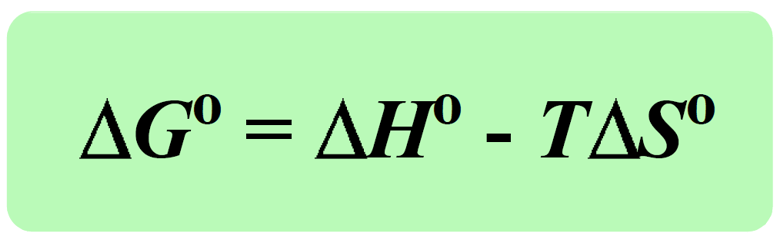The Effect of 𝚫H, 𝚫S, and T on 𝚫G – Spontaneity
We have seen in the previous post, that the Gibbs free energy of a reaction is a comprehensive measure of the spontaneity of the reaction that can be calculated based on the parameters of the system. Today, we … Read more