First, we need to draw the Lewis structure of CH3+.
In short, these are the steps you need to follow for drawing a Lewis structure:
1. Write the correct skeletal structure for the molecule.
* Hydrogen atoms are always terminal (only one bond)
* Put more electronegative elements in terminal positions
2. Sum the valence electrons from all the atoms.
3. Use a pair of electrons to form a bond between each pair of bound atoms.
4. Add the remaining electrons to satisfy the octet for a more electronegative atom first.
5. If any atoms lack an octet, make a double or triple bond to give them an octet.
The methyl cation (CH3+) has 4 + 3 – 1 = 6 valence electrons. We subtract one because there is a formal charge of 1+. All the six electrons are used to make the three C-H bonds, so we can show the Lewis structure of CH3+ as:
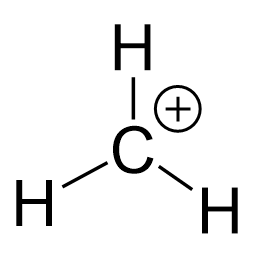
We can confirm the formal charge of carbon by using the formula:
FC= V – (N + B)
Where:
V – number of valence electrons
N – number of nonbonding electrons
B – number of bonds
So, the formal charge of the oxygen will be
FC (C) = 4 – (0 + 3) = +1
The steric number of carbon is three which corresponds to sp2 hybridization, and both electron and molecular geometries are trigonal planar.
Check this 99-question multiple-choice quiz on Geometry and Hybridization:
Geometry and Hybridization Quiz
Check Also

