Percentage composition shows the amount of each element in a compound expressed as a mass percent.
To calculate the percentage of an element, multiply its molar mass and the subscript which shows how many moles of it are present in one mole of the compound, then divide the product by the molar mass of the compound.
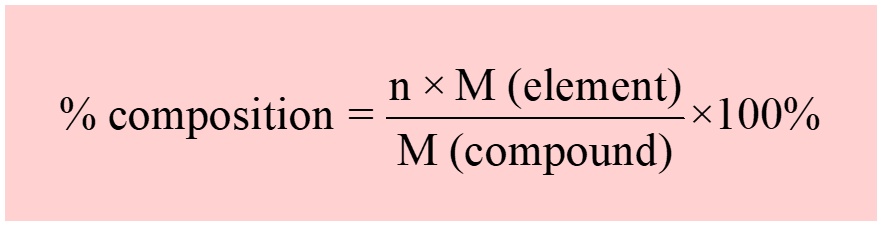
For example, let’s determine the percent composition of sulfuric acid (H2SO4). If the element is not specified, we need to calculate the percentage of all the elements.
The molar mass of sulfuric acid is: M (H2SO4) = 2 x 1.0 + 32 + 4 x 16.0 = 98 g/mol.
\[{\rm{\% }}\;{\rm{(H)}}\;{\rm{ = }}\;\frac{{{\rm{2}}\;{\rm{ \times }}\,{\rm{M}}\,{\rm{(H)}}}}{{{\rm{M}}\;{\rm{(}}{{\rm{H}}_{\rm{2}}}{\rm{S}}{{\rm{O}}_{\rm{4}}}{\rm{)}}}}\;{\rm{ = }}\;\frac{{{\rm{2}}\,{\rm{ \times }}\,{\rm{1}}{\rm{.0}}\,}}{{{\rm{98}}\,}}\,{\rm{ \times }}\,100\% \; = \;2.0\% \]
\[{\rm{\% }}\;{\rm{(S)}}\;{\rm{ = }}\;\frac{{{\rm{M}}\,{\rm{(S)}}}}{{{\rm{M}}\;{\rm{(}}{{\rm{H}}_{\rm{2}}}{\rm{S}}{{\rm{O}}_{\rm{4}}}{\rm{)}}}}\;{\rm{ = }}\;\frac{{{\rm{32}}{\rm{.1}}\,}}{{{\rm{98}}\,}}\,{\rm{ \times }}\,100\% \; = \;33\% \]
\[{\rm{\% }}\;{\rm{(O)}}\;{\rm{ = }}\;\frac{{{\rm{4}}\;{\rm{ \times }}\,{\rm{M}}\,{\rm{(O)}}}}{{{\rm{M}}\;{\rm{(}}{{\rm{H}}_{\rm{2}}}{\rm{S}}{{\rm{O}}_{\rm{4}}}{\rm{)}}}}\;{\rm{ = }}\;\frac{{{\rm{4}}\,{\rm{ \times }}\,{\rm{16}}\,}}{{{\rm{98}}\,}}\,{\rm{ \times }}\,100\% \; = \;65\% \]
The sum of the percentages of all elements in the compound is 100%, so you can find the last one by subtracting the sum of the other elements from 100. This relies on the accuracy of the previous calculations, so double-check your calculation!
For example, let’s determine the percent composition of ammonia (NH3).
\[{\rm{\% }}\;{\rm{(H)}}\;{\rm{ = }}\;\frac{{{\rm{3}}\;{\rm{ \times }}\,{\rm{M}}\,{\rm{(H)}}}}{{{\rm{M}}\;{\rm{(N}}{{\rm{H}}_{\rm{3}}}{\rm{)}}}}\;{\rm{ = }}\;\frac{{{\rm{3}}\,{\rm{ \times }}\,{\rm{1}}{\rm{.0}}\,}}{{{\rm{17}}\,}}\,{\rm{ \times }}\,100\% \; = \;17.6\% \]
Therefore, nitrogen makes 100 – 17.6 = 82.4% of ammonia by mass.
Empirical Formula
The empirical formula of a compound indicates the mole ratio of atoms in the simplest whole numbers. For example:
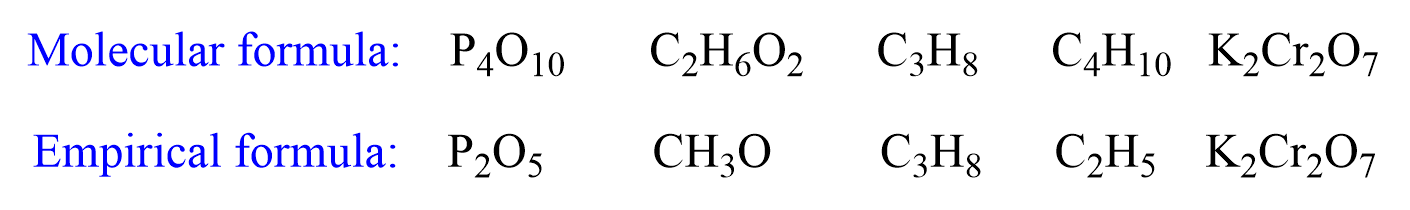
Notice that the empirical formula can be the same as the molecular formula if the numbers cannot be simplified any further.
Determining the Empirical and Molecular Formulas
To determine the empirical and molecular formulas, given the mass or percentage of the elements, you need to convert them to moles. This is because, remember, the formula of a compound tells us how many moles of each element there are in one mole of that compound.
For example, the formula of fructose C6H12O6 indicates that in one mole of fructose, there are six moles of carbon, twelve moles of hydrogen, and six moles of oxygen.
Therefore, if you are asked to determine the empirical or molecular formula of a compound, you need to find the moles of each element.
For example, What are the empirical and molecular formulas of ascorbic acid if it consists of 40.92% carbon (C), 4.58% hydrogen (H), and 54.50% oxygen (O) and the molar mass is 176.1 g/mol?
The quantities are given in percentage, so we’ll go ahead and write these numbers as subscripts in the formula and convert them to moles following the steps in this plan:
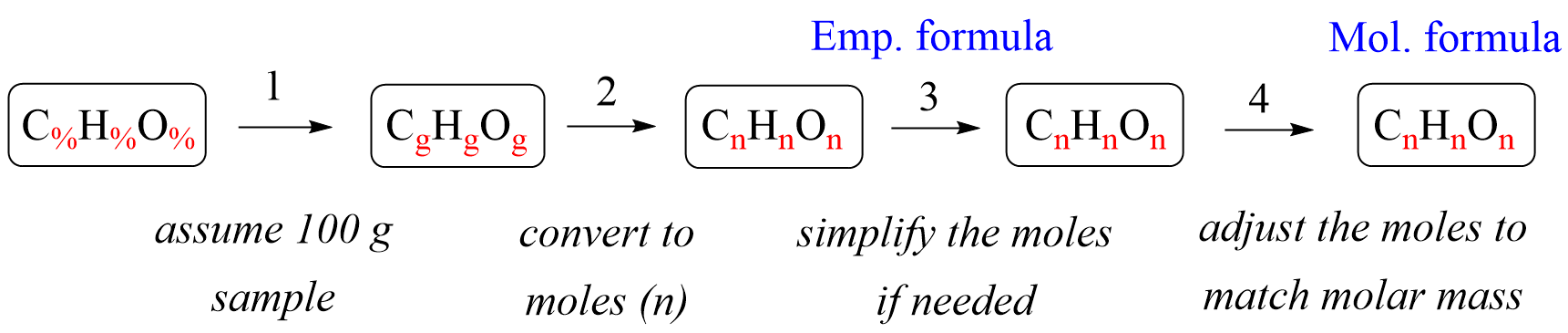
1) The first step is converting the % to mass, and you may be wondering how to achieve this transformation. Remember, whenever the quantities are not given or not relatable, you can assume a certain amount of the compound because whether it is, for example, 100 g or 450 g of water, the formula is always the same.
Now, when going from % to mass, the easiest is to assume a 100 g sample since this converts to units from % to grams without changing the numbers. So, assuming a 100 g sample, we have 40.92% g C, 4.58% g H, and 54.50% g O.
2) Find the moles of C, H, and O using their molar masses:
\[{\rm{n}}\;{\rm{(C)}}\;{\rm{ = }}\,\frac{{{\rm{m}}\;}}{{{\rm{M}}\;}}\,{\rm{ = }}\;\frac{{{\rm{40}}{\rm{.92}}\;{\rm{g}}\;}}{{{\rm{12}}{\rm{.0}}\;{\rm{g/mol}}\;}}\;{\rm{ = }}\,{\rm{3}}{\rm{.41}}\;{\rm{mol}}\]
\[{\rm{n}}\;{\rm{(H)}}\;{\rm{ = }}\,\frac{{{\rm{m}}\;}}{{{\rm{M}}\;}}\,{\rm{ = }}\;\frac{{{\rm{4}}{\rm{.58}}\;{\rm{g}}\;}}{{{\rm{1}}{\rm{.00}}\;{\rm{g/mol}}\;}}\;{\rm{ = }}\,{\rm{4}}{\rm{.58}}\;{\rm{mol}}\]
\[{\rm{n}}\;{\rm{(C)}}\;{\rm{ = }}\,\frac{{{\rm{m}}\;}}{{{\rm{M}}\;}}\,{\rm{ = }}\;\frac{{{\rm{54}}{\rm{.50}}\;{\rm{g}}\;}}{{{\rm{16}}{\rm{.0}}\;{\rm{g/mol}}\;}}\;{\rm{ = }}\,{\rm{3}}{\rm{.41}}\;{\rm{mol}}\]
Don’t worry about significant figures here and keep at least three decimals.
3) Dividing the numbers by the smallest one is usually a good start to simplifying them. So, dividing by 3.41, the moles are:
C1H1.343O1
We still need to simplify the moles of H. Remember, you can divide or multiply by any number to get a set of the simplest integers. Multiplying 1.343 by 3 is 4.029, which is approximately equal to 4 (4.029 ≈ 4). Make sure to multiply all the subscripts – you cannot multiply only one and leave the others because that would change the mole ratio of the elements. Therefore, by multiplying all the subscripts by 3, we get that the empirical formula is
C3H4O3
4) Next, we need to check if the empirical formula is the same as the molecular formula of the compound. For this, calculate the molar mass of the empirical formula C3H4O3, to see if it matches the actual molar mass given in the problem (176.1 g/mol):
3 x 12.0 + 4 x 1.00 + 3 x 16.0 = 88 g/mol
This indicates that the empirical formula of this compound is not the same as its molecular formula.
Divide 176.1 by 88 to see how many times larger the molar mass of the molecular formula is compared to the one of the empirical formula:
176.1/88 ≈ 2
Therefore, the subscripts (moles) in the empirical formula must be multiplied by two to obtain the molecular formula:
molecular formula = 2 x empirical formula
2 x C3H4O3 = C6H8O6
Calculate the molar mass of this formula to make sure it matches the one given in the problem:
M (C6H8O6) = 6 x 12.0 + 8 x 1.00 + 6 x 16.0 = 176 g/mol
Check Also
- The Mole and Molar Mass
- Molar Calculations
- Percent Composition and Empirical Formula
- Stoichiometry of Chemical Reactions
- Limiting Reactant
- Reaction/Percent Yield
- Stoichiometry Practice Problems
Practice
Calculate the percentage composition of butane, C4H10. How many percent of carbon and hydrogen, by mass, does butane contain?
What is the percent composition of lithium in Li3PO4 ?
What is the percent composition of water in Cupric sulfate pentahydrate, CuSO4·5H2O ?
How many grams of phosphorous are there in a 49.7 g sample of P2O5?
Determine the empirical formula for a compound that contains 18.75 % C, 6.25% H, and 75% O by mass.
Fructone is used in different types of perfumes as a synthetic fruit-aroma additive. It contains 55.17 % C, 8.046 % H and the rest being oxygen. Determine the empirical and molecular formula of Fructone considering that its molar mass is 174.0 g/mol.
What is the mass percent of the carbon in ethanol, C2H5OH?
Calculate the percent composition by mass of the steroid hormone cortisol with the molecular formula of C21H30O5.
Determine the molecular formula of each compound given the empirical formula.
(a) Empirical formula CH2 (M = 84 g/mol)
(b) Empirical formula NH2 (M = 80 g/mol)
(c) Empirical formula CHS (M = 180.4 g/mol)
Determine the empirical formula of the compound that is formed when 0.251 mol of nitrogen atoms are combined with 0.6273 mol of oxygen atoms.
In a reaction, 0.61764 g of carbon was combined with 7.309 g of chlorine. What is the empirical formula of the resulting product?
Determine the molecular formula of a compound that contains 34.2% C, 11.43% O, 22.0% P, 7.17% H, and 25.2% Cl, and has a molar mass of 140.55 g/mol.
Nicotine contains 74.0% carbon, 8.7% hydrogen, and 17.3% nitrogen by mass. What are the empirical and molecular formulas of nicotine considering its molar mass is 162.1 g/mol.
Cysteine is an important non-essential amino acid that contains 29.74 % carbon, 5.82 % hydrogen, 26.41 % oxygen,11.56 % nitrogen, and 26.47 % sulfur. What are the empirical and molecular formulas of cysteine considering it molar mass is 121.2 g/mol.
Muscle soreness during excessive physical activity is due to the synthesis of lactic acid that contains 40.0 % C, 6.71 % H, and 53.3 % O by mass. Determine the empirical and molecular formulas of lactic acid considering it molar mass is 90.08 g/mol.
Acetone is commonly used as a nail polish remover. When 0.2647 g of acetone was burned in a combustion apparatus, 0.6019 g of CO2 and 0.4925 g of H2O were formed. Determine the molecular formula of acetone if its molas mass is 58.08 g/mol.

Hi, I have a question. Why did you multiply 3 to 1.343 for Hydrogen? I am confused of where that 3 came from.
Hi,
So, the idea of getting the empirical formula is to divide or multiply the subscripts by any number to get whole numbers. The 3s of C and O are coming from multiplying their subscripts by 3 which makes the H equal to 4 since we have to multiply/divide the moles of all the elements in the formula.