In this post, we will determine the oxidation numbers in hydroxylamine, NH2OH.
To determine the oxidation number of an atom(s) in a molecule or an ion, start with the known oxidation numbers and the rules summarized below:
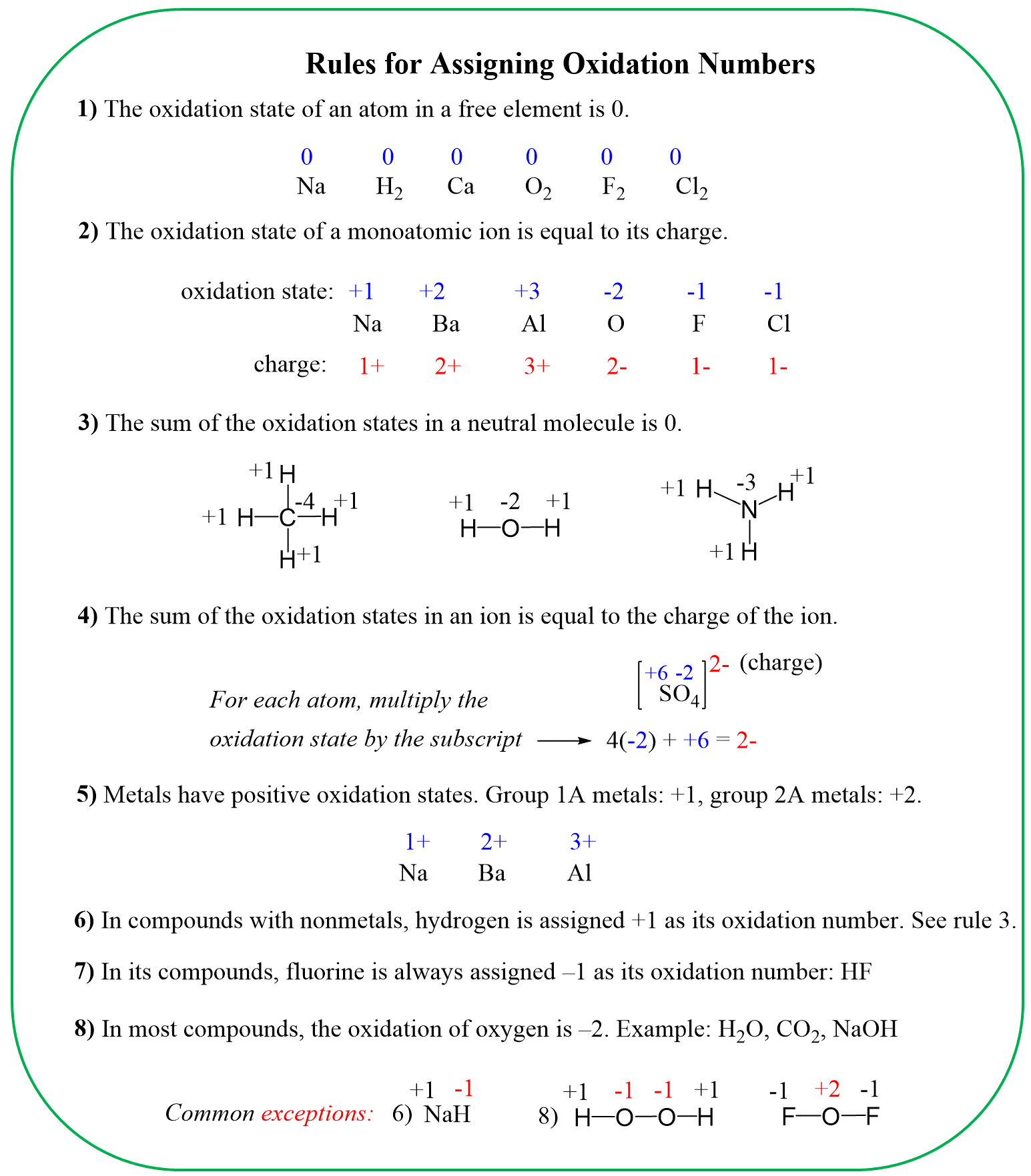
Keep in mind that the summary is zero for neutral molecules and is equal to the charge for ions.
For NH2OH, the elements with standard oxidation states are oxygen (-2), and hydrogen (+1), so we need to determine the oxidation state of the nitrogen.
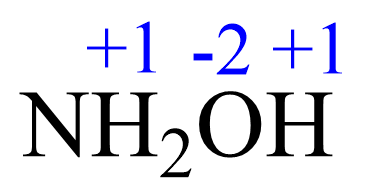
Assign x for the oxidation state of N and set up an equation:
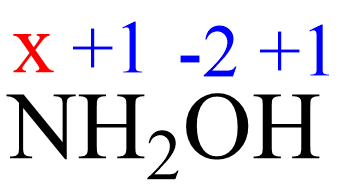
1(x) + 2(+1) + 1(-2) + 1(+1)= 0
x + 2 -2 +1= 0
x = -1
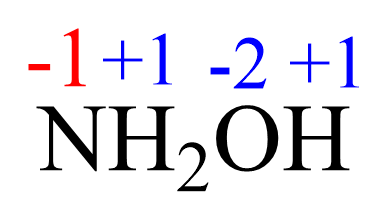
We can also assign the oxidation state of the hydroxide ion -1 as if it is a monoatomic ion (rule 2), and set up another equation:
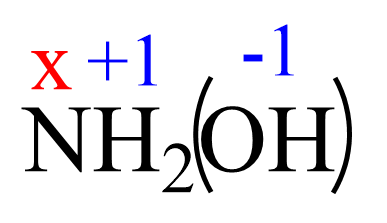
1(x) + 2(+1) + 1(-1) = 0
x = -1
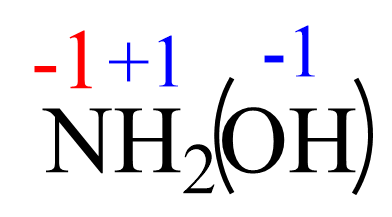
Further Reading
- Solutions
- Strong and Weak Electrolytes
- Dissociation of Ionic Compounds
- Molecular, Ionic, and Net Ionic Equations
- Molarity
- Dilution
- Ion Concentration
- Precipitation Reactions
- Definitions of Acids and Bases
- Naming Acids and Bases
- Acid-Base Reactions
- Displacement Reactions
- Predicting The Products of Chemical Reactions
- Stoichiometry of Reactions in Aqueous Solutions
- Acid-Base Titrations
- Oxidation State
- Oxidation-Reduction (Redox) Reactions
More examples of the oxidation state in this multiple-choice quiz:

