Practice problems on the colligative properties of solutions covering the freezing point depression, boiling point elevation, vapor pressure, and osmotic pressure of solutions prepared with nonelectrolytes as well as ionic compounds.
The links s for the corresponding topics are given herein:
Practice
Vapor Pressure Lowering
Calculate the vapor pressure of a solution at 25°C that is made by adding 47.9 g of glucose (C6H12O6) to 340.0 mL of water. The vapor pressure of pure water at 25°C is 23.8 torr and the density of water is 1.00 g/mL.
Calculate the vapor pressure of a solution prepared by adding 128 g glycerin (C3H8O3) to 421 g of water at 35.0 oC. Assume that the vapor pressure of pure water at this temperature is 42.2 torr and glycerin is a nonvolatile and nonelectrolyte liquid.
Calculate the vapor pressure and the vapor pressure lowering of the solution at 25°C prepared by dissolving 26.7 g of sucrose, C12H22O11, in 85.0 g of water. The vapor pressure of pure water at 25°C is 23.8 torr.
How many grams of urea (NH2)2CO) must be added to 485 g of water to prepare a solution with a vapor pressure of 22.0 torr at 25 oC? The vapor pressure of water at 25 °C is 23.8 torr.
The vapor pressure of a solution containing 60.0 g naphthalene (C10H8) in 245 g benzene (C6H6) is 130. torr at 35 oC. Calculate the vapor pressure of pure benzene at this temperature considering that naphthalene is a nonelectrolyte solid.
Determine the mole fraction of the solute in a solution of methanol with a vapor pressure of 675 torr at 64.7 °C which is the normal boiling point of methanol.
Determine the molar mass of a nonvolatile, nondissociating compound if adding 15.7 g of it to 92.0 g of ethanol (C2H5OH) has decreased the vapor pressure from 0.875 atm to 0.842 atm.
How many moles of a nonvolatile compound was added to 0.400 mol of benzene, C6H6 at 25°C if the resulting solution has a vapor pressure of 71.0 Torr? The vapor pressure of pure benzene at this temperature is 94.6 Torr.
A solution is prepared by mixing 4.23 g of chloroform (CHCl3) and 3.68 g of hexane (C6H14) at 25 °C. The vapor pressures of pure chloroform and pure hexane, at this temperature, are 197 torr and 154 torr, respectively. Assuming ideal behavior, calculate the total vapor pressure above the solution.
A solution contains a mixture of pentane, C5H12 and diethyl ether, (C2H5)2O. At a certain temperature, the vapor pressure of the solution is 438 torr. At this temperature, pure pentane and diethyl ether have vapor pressures of 362 torr and 512 torr, respectively. What is the mole fraction composition of the mixture assuming an ideal behavior?
A solution is prepared by mixing 5.81 g acetone, C3H6O and 11.9 g chloroform, CHCl3. Determine if this is an ideal solution given that at 35 °C the total vapor pressure is measured to be 260. torr. The vapor pressures of pure acetone and pure chloroform at 35 °C are 345 and 293 torr, respectively.
Freezing Point and Melting Point
Using the appropriate data in the table, determine the freezing point depression of the solution that contains 24.1 g urea (NH2)2CO) in 485 mL of water.
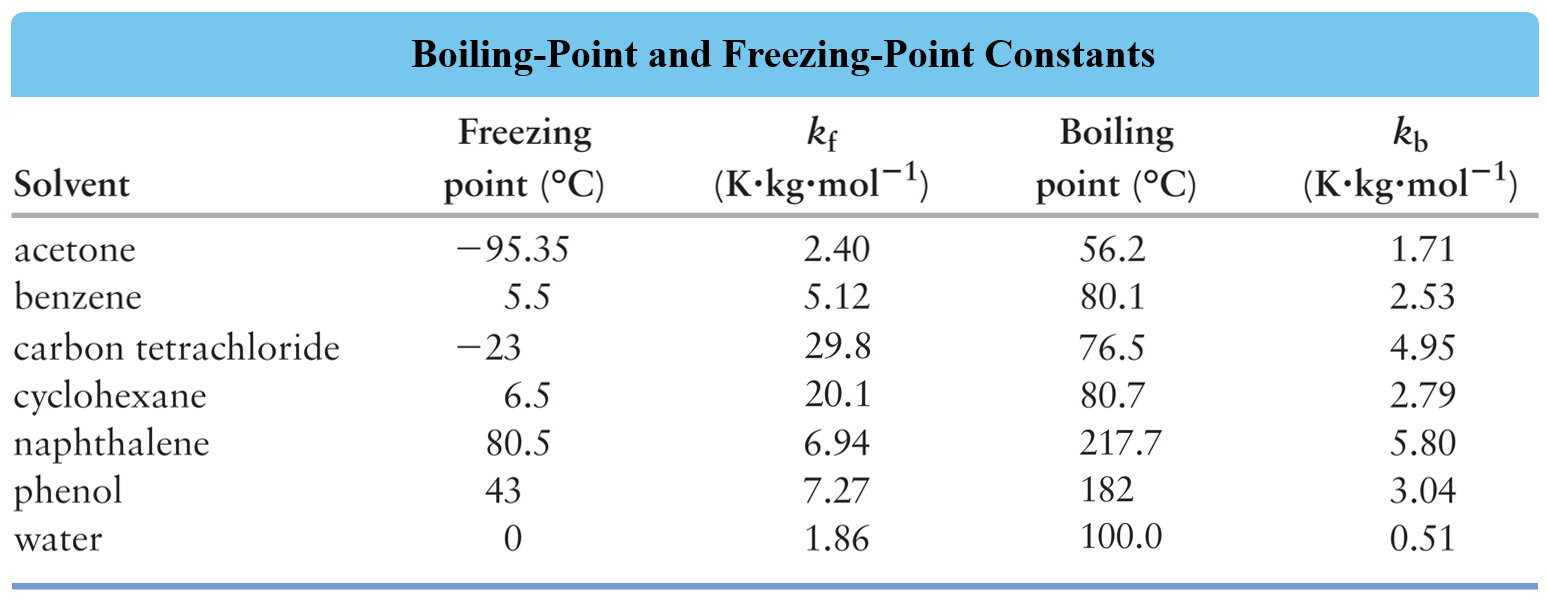
Determine the freezing point of a solution containing 1.80 g of glycerol, C3H8O3, in 26.0 g of water.
A solution is prepared by dissolving 75.0 g glycerin (C3H8O3) in 240. g water. Calculate the boiling point of the solution. Glycerin is a nonelectrolyte.
A 1.60 g sample of a newly synthesized supplement was dissolved in 12.0 g carbon tetrachloride, CCl4. The boiling point of this solution was determined to be 79.2 oC while the boiling point of pure carbon tetrachloride is 76.5 oC. Calculate the molar mass of the supplement considering that is a nonelectrolyte.
A 12.4 g of an unknown nonelectrolyte was dissolved in 100.0 g of water to prepare a solution with a freezing point of -1.7 °C. Calculate the molar mass of the unknown compound.
How many grams of ethylene glycol (C2H6O2), a nonelectrolyte, must be added to 5.40 L water to prepare an antifreeze solution with a freezing point of -30.0 oC?
A sample of 23.0 g naphthalene (C10H8) was added to benzene (C6H6) and the resulting solution had a boiling point of 83.7 oC. How many liters of benzene were used to prepare the solution if the normal boiling point of benzene is 80.1 oC and the density is 0.877 g/mL?
You are planning a winter trip and you have an antifreeze solution that is 40.0 % by mass of ethylene glycol (C2H6O2) in water. Would this antifreeze be good enough for the trip considering that the temperature goes down as far as -30.0 °C? Ethylene glycol is a nonelectrolyte.
Calculate the osmotic pressure of the solution containing 3.52 g of urea (NH2)2CO) in 485 mL of solution at 298 K.
How would you prepare 1.0 L of an aqueous solution of sucrose (C12H22O11) having an osmotic pressure of 8.5 atm at 25 oC?
What is the average molecular mass of a nonelectrolyte biopolymer if dissolving 68.4 g of it in 1.25 L of water, produces a solution with an osmotic pressure of 2.4 atm at 25 oC.? Assume no volume change when the polymer is added.
Properties of Electrolyte Solutions
A solution is prepared by dissolving 0.248 mol of NaCl in 1.85 mol of H2O. Calculate the vapor pressure of the solution at 40 °C. The vapor pressure of pure water at 40 °C is 55.0 torr.
Calculate the vapor pressure of a solution prepared by dissolving 46.0 g of K2SO4 in 200. g water at 25 °C. The vapor pressure of pure water at 25 °C is 23.8 torr.
Calculate the freezing point of the solution prepared by dissolving 4.80 g NaCl in 25.0 g of water.
Calculate the boiling point of the solution prepared by dissolving 5.4 g FeCl3 in 36.0 g of water.
How many grams of NaCl were added to 1.00 L of water if the resulting solution has a freezing point of -7.40 °C? The density of water is 1.00 g/mL.
Calculate the osmotic pressure of a solution prepared by dissolving 26.8 g of MgCl2 in 600. mL of water at 298 K. Assume the volume is not changing and the van’t Hoff factor for MgCl2 is 3.

