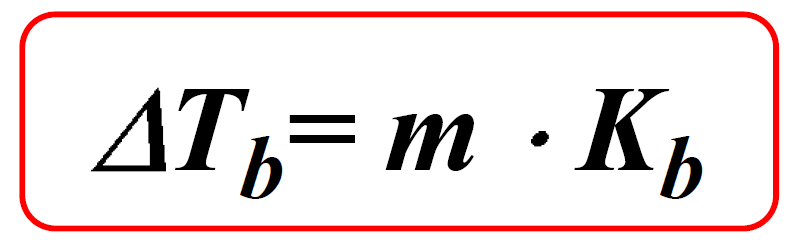Properties of Solutions
Osmotic Pressure
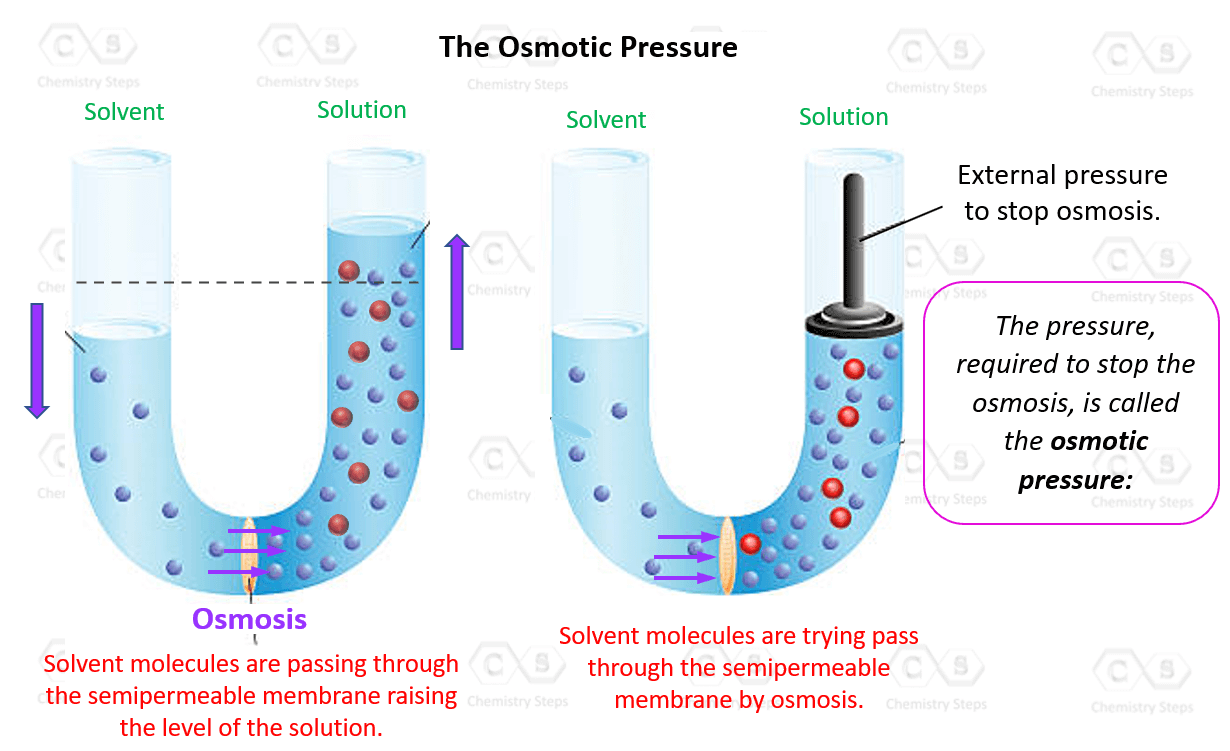
Another colligative property of solutions is the osmotic pressure. So, what is osmotic pressure? To understand it, let’s imagine a tube with a semipermeable membrane that separates a solvent from a solution: The semipermeable membrane allows passing of … Read more
Freezing Point Depression
Have you ever wondered what they are sparing on airplanes on cold days before they take off? What they are spraying is a solution typically containing ethylene glycol, C₂H₆O₂ which lowers the freezing point of water. It is essentially … Read more
Boiling Point Elevation
In the previous post, we have seen that solutions have lower vapor pressure than the corresponding pure solvents. In short, this is because the solution has higher entropy than the solvent, and the evaporation of the solvent molecules from the … Read more
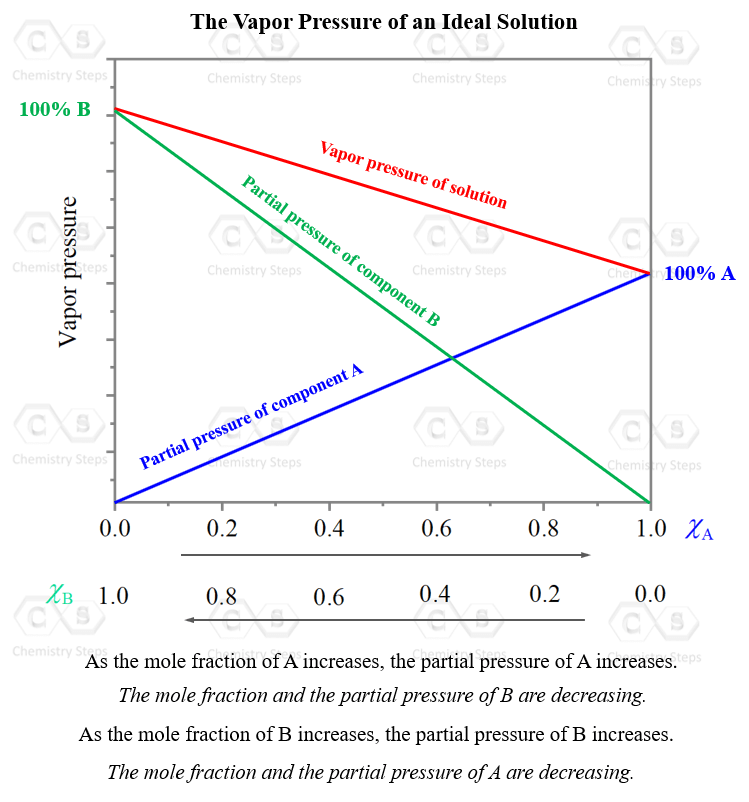
Vapor Pressure Lowering
Vapor pressure lowering is a colligative property of solutions. So, first, what is vapor pressure? Recall that when a liquid evaporates in a closed container, an equilibrium is established between the liquid and the gas, and the pressure exerted by the … Read more
Colligative Properties
Colligative (collective) properties are those that depend only on the number of particles dissolved in solution, and not on the type of particle. For example, salt is sometimes scattered on icy roads to melt the ice. What happens … Read more
Colligative Properties – Practice Problems
Practice problems on the colligative properties of solutions covering the freezing point depression, boiling point elevation, vapor pressure, and osmotic pressure of solutions prepared with nonelectrolytes as well as ionic compounds. The links s for the corresponding topics are given … Read more