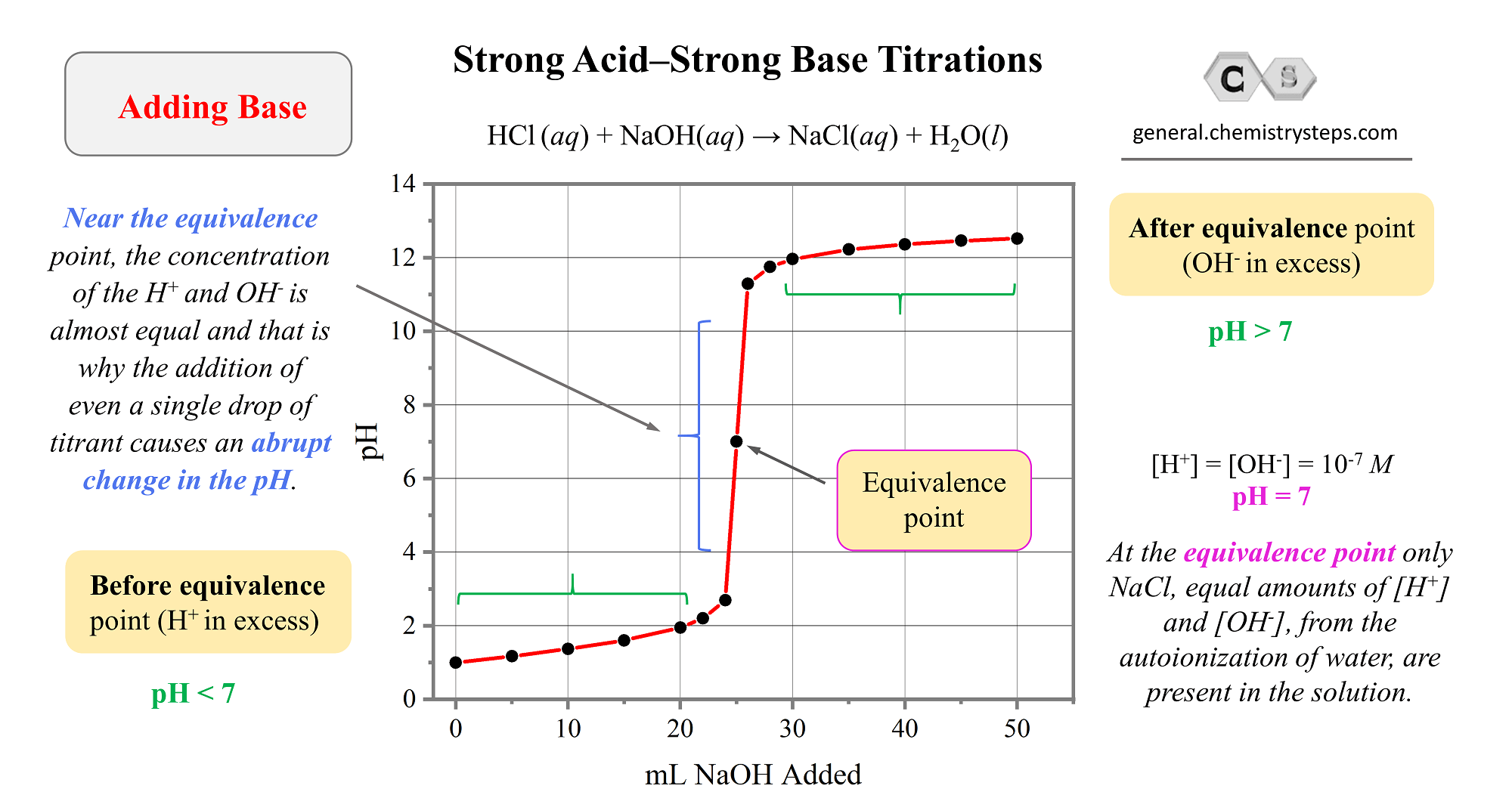
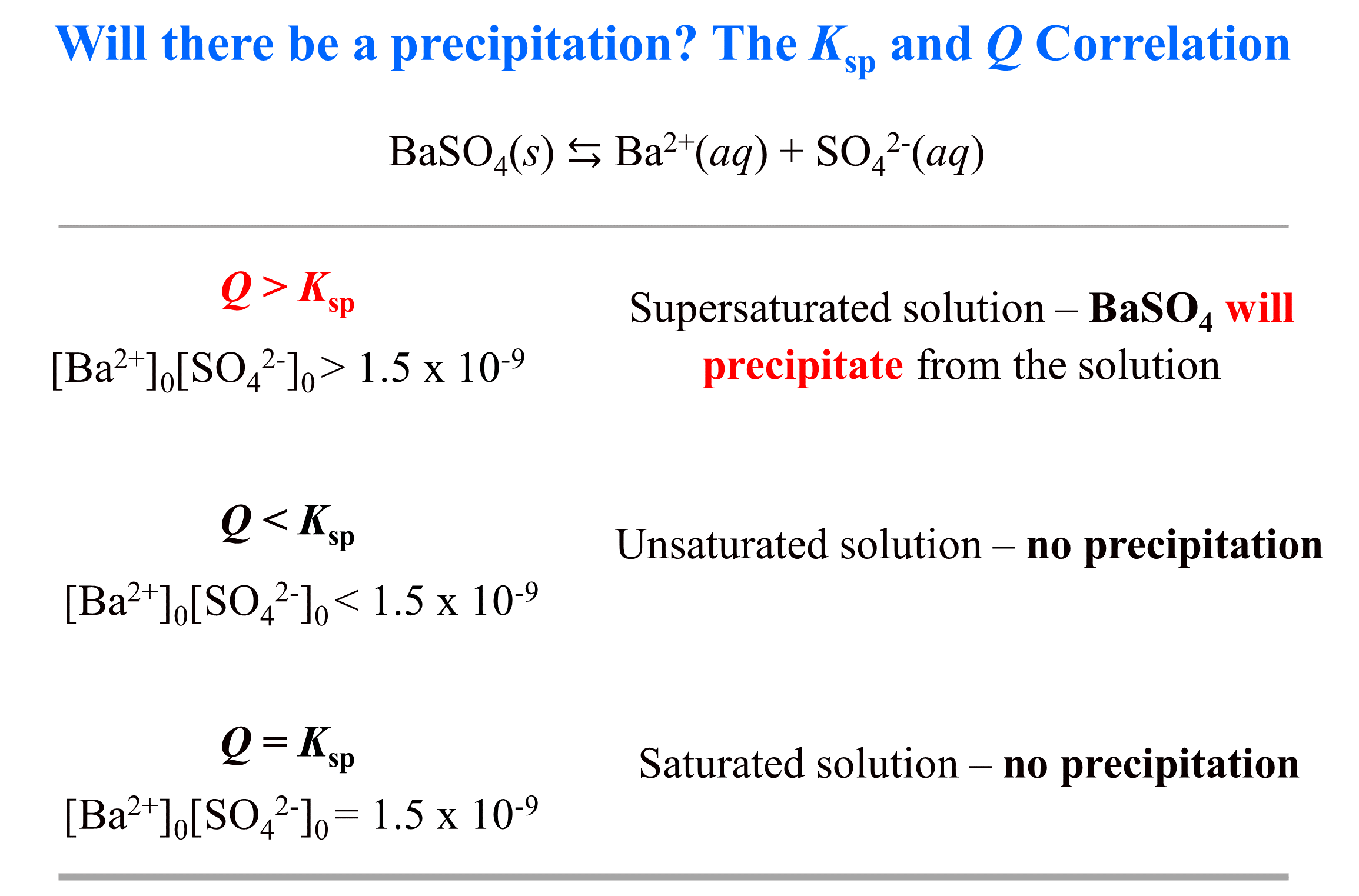
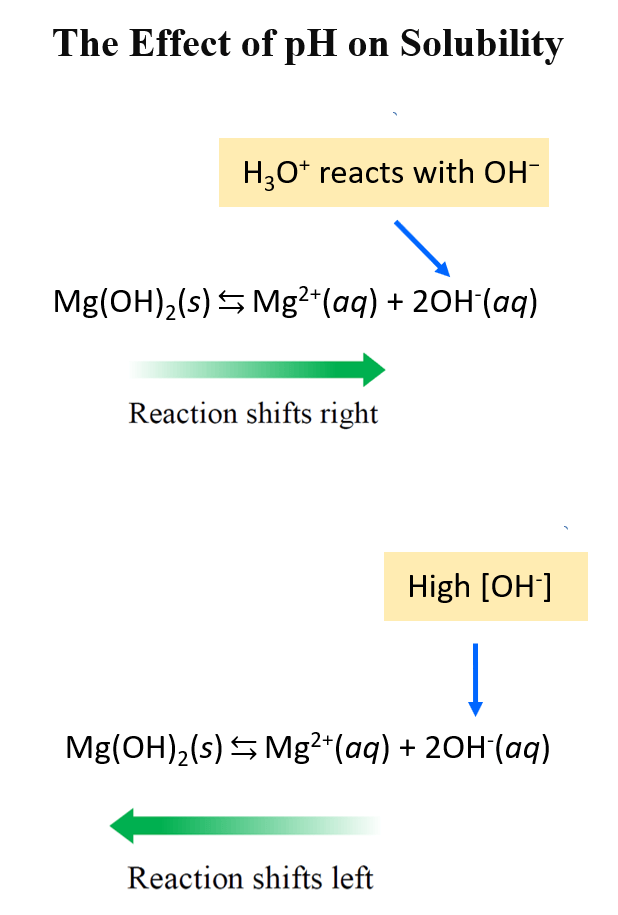
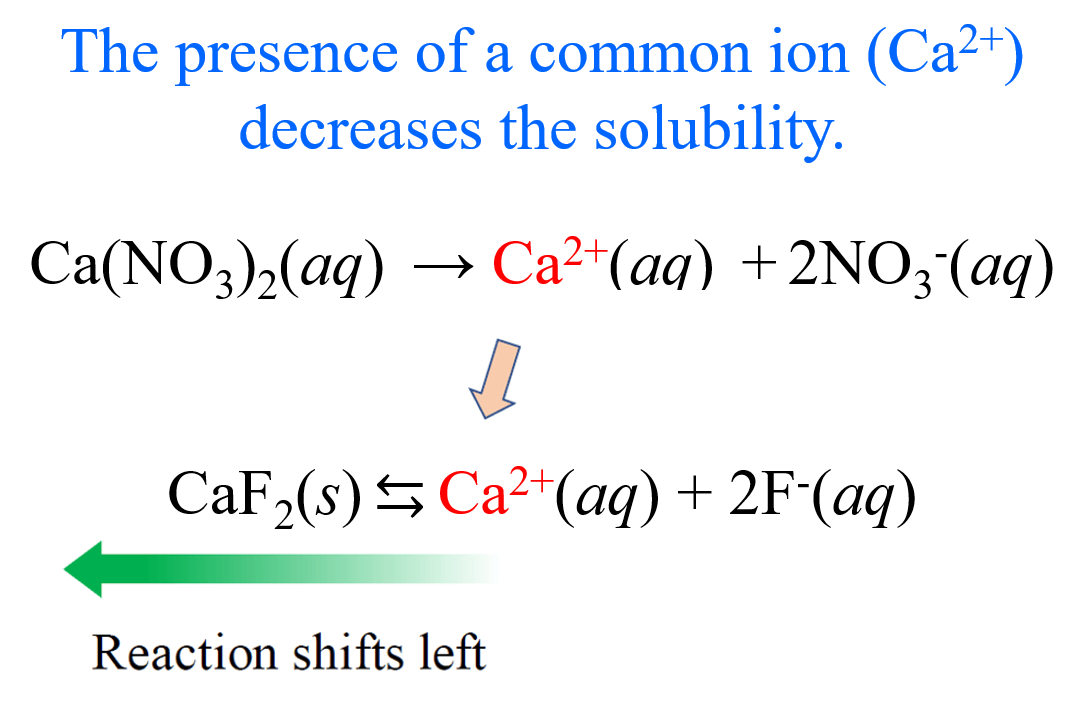Strong Acid–Strong Base Titrations
Earlier in the semester, or perhaps in your Chem II class, you learned about acid-base titrations which were used to determine the concentration of an acid or a base. For this, we are using a standard solution (titrant) which is … Read more