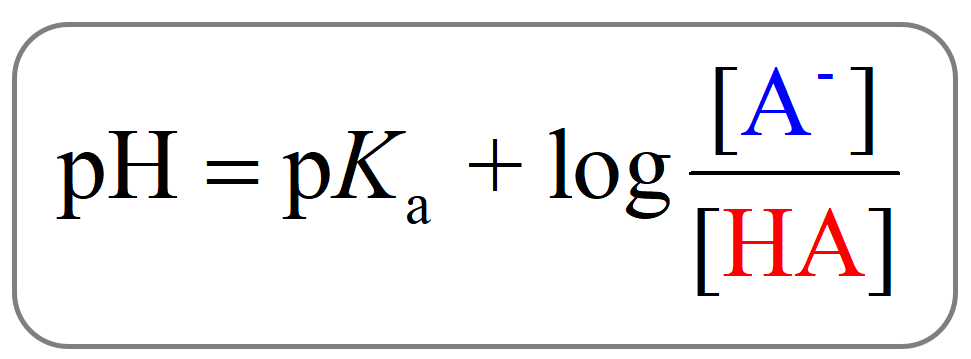pH and pKa Relationship
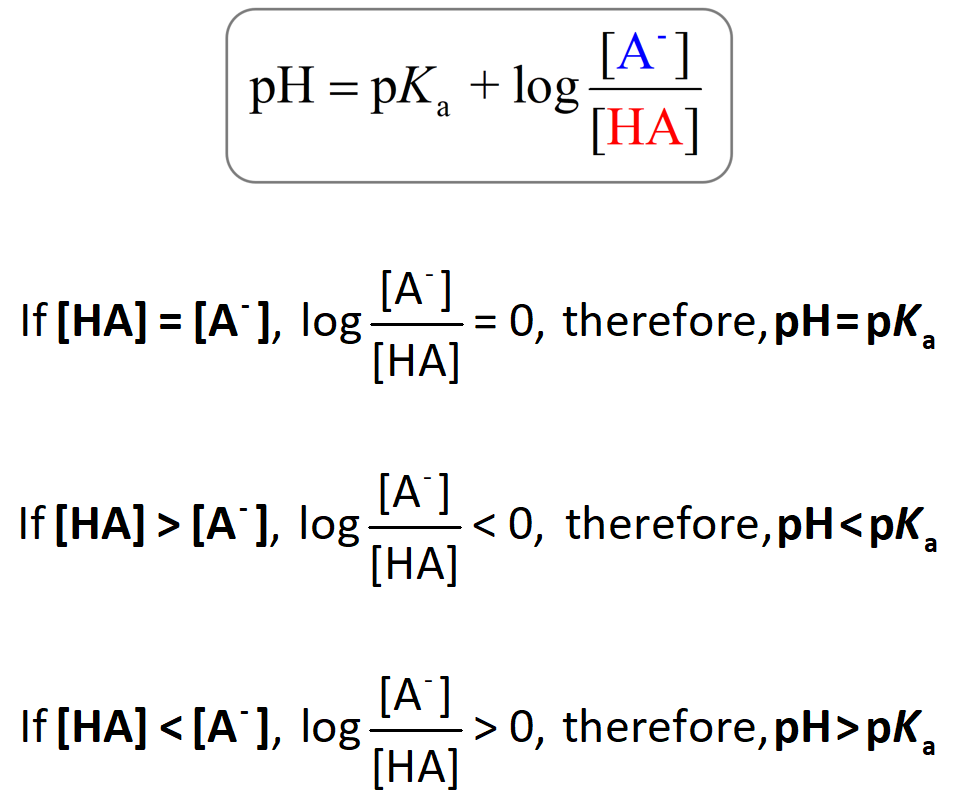
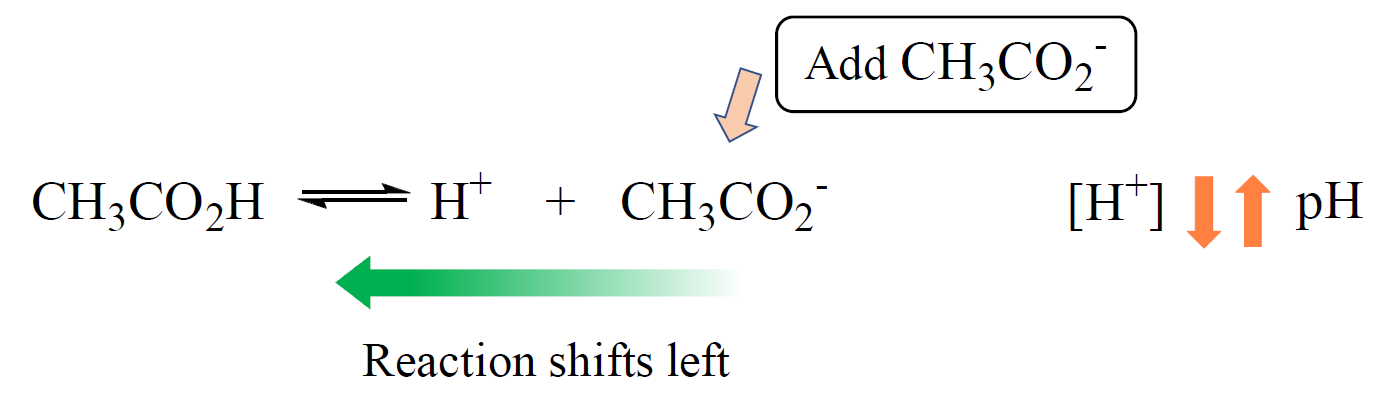
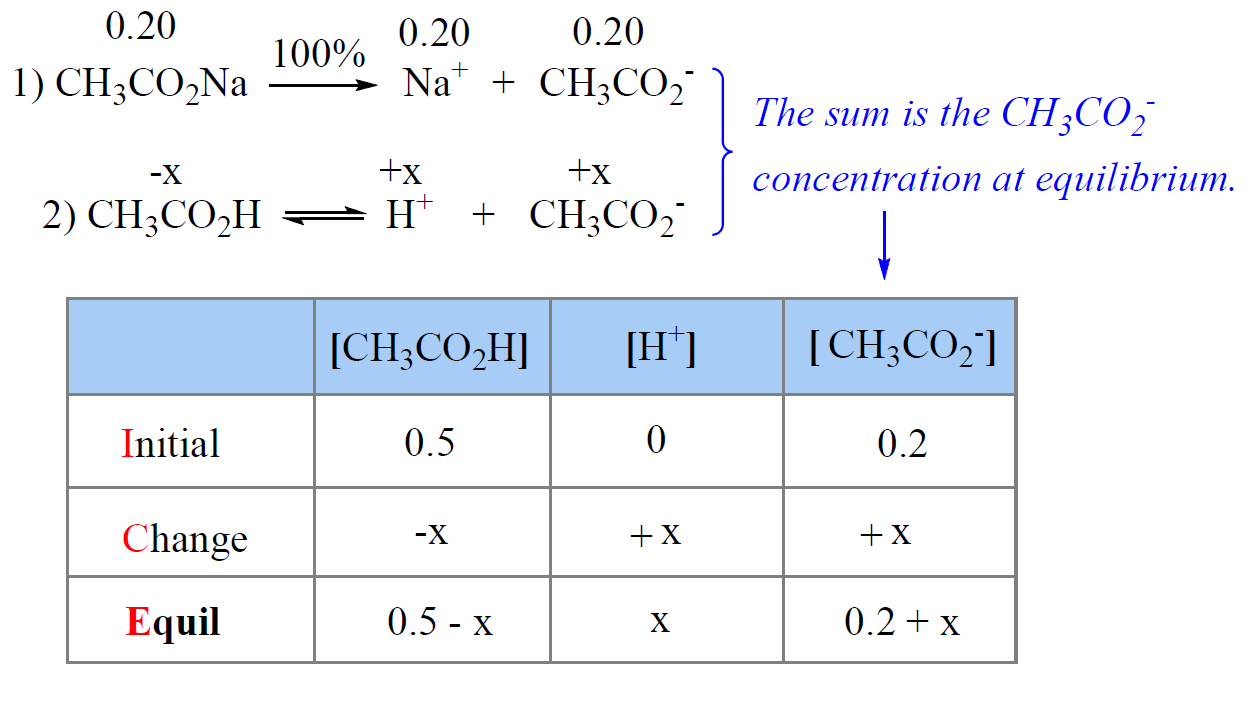
The Henderson–Hasselbalch equation shows the correlation of the pKa and pH of the buffer solution. The pKa of a given acid, or the conjugate acid of the base, is constant and therefore, the only variable in the equation is the … Read more