Sulfur is the central atom:
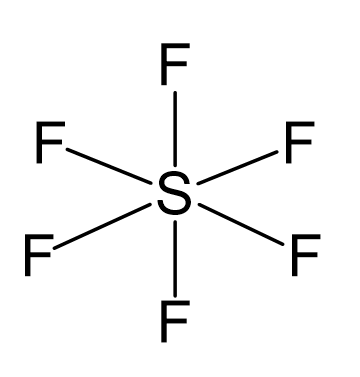
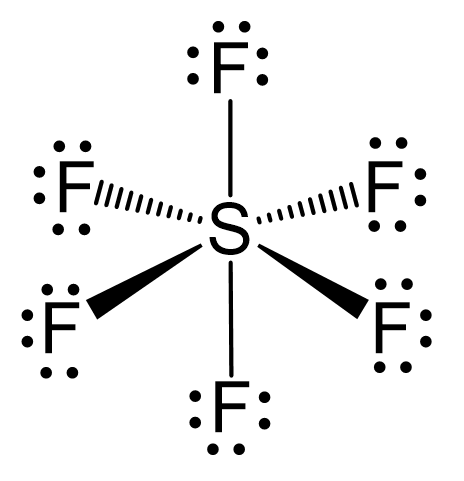
There are 6 + 6×7 = 48 valence electrons, and 12 are taken for making 6 covalent bonds. Each fluorine takes three lone pairs, so there are no electrons left: 48 – (12 + 6×6) = 0.
The central atom has 6 atoms connected to it, and no lone pairs, therefore, both geometries are octahedral:
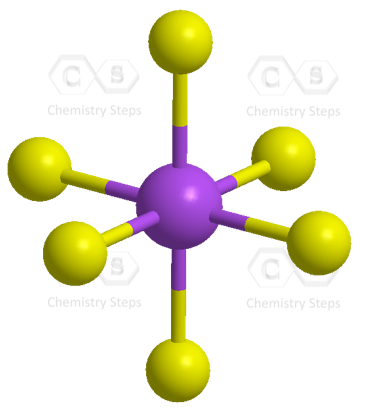
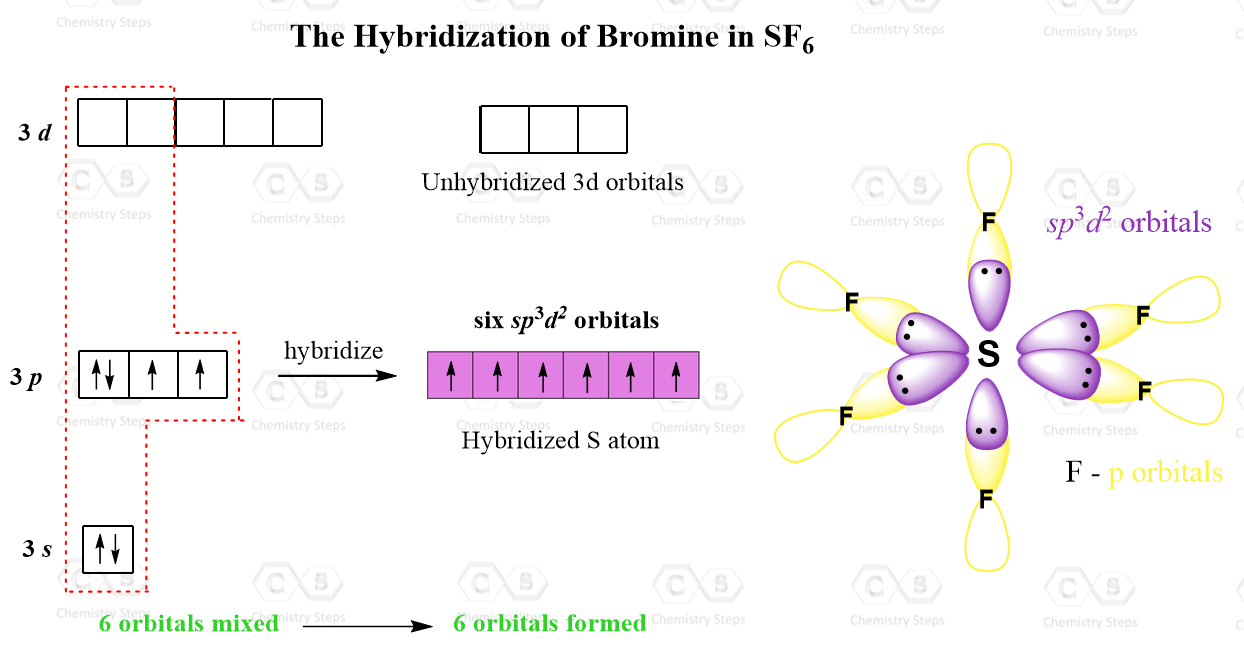
There are 6 units (atoms and lone pairs) on the central atom, and to accommodate them, it needs 6 orbitals which is achieved through sp3d2 hybridization.
Check this 99-question multiple-choice quiz on Geometry and Hybridization:
Free
Geometry and Hybridization Quiz
Check Also

