There are a few definitions of acids and bases which we covered in an earlier post. Although it is not always accurate, you can recognize acids when you see an H with any of the anions you learned so far. For example, HCl, H2SO4, HNO3, and H3PO4.
The name of an acid is derived from the name of its anion. These are the correlation patterns for the names of monoatomic and polyatomic (oxoanions) ions and their acids:
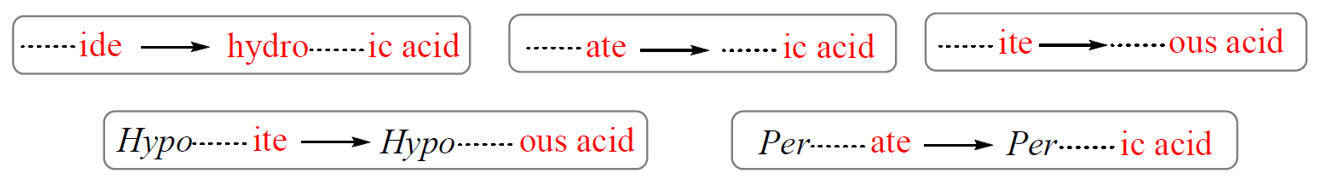
For example,
Cl– (chloride) – HCl (hydrochloric acid)
NO3– (nitrate) – HNO3 (nitric acid)
SO32- (sulfite) – H2SO3 (sulfurous acid)
ClO– (hypochlorite) – HClO (hypochlorous acid)
ClO4– (perchlorate) – HClO4 (perchloric acid)
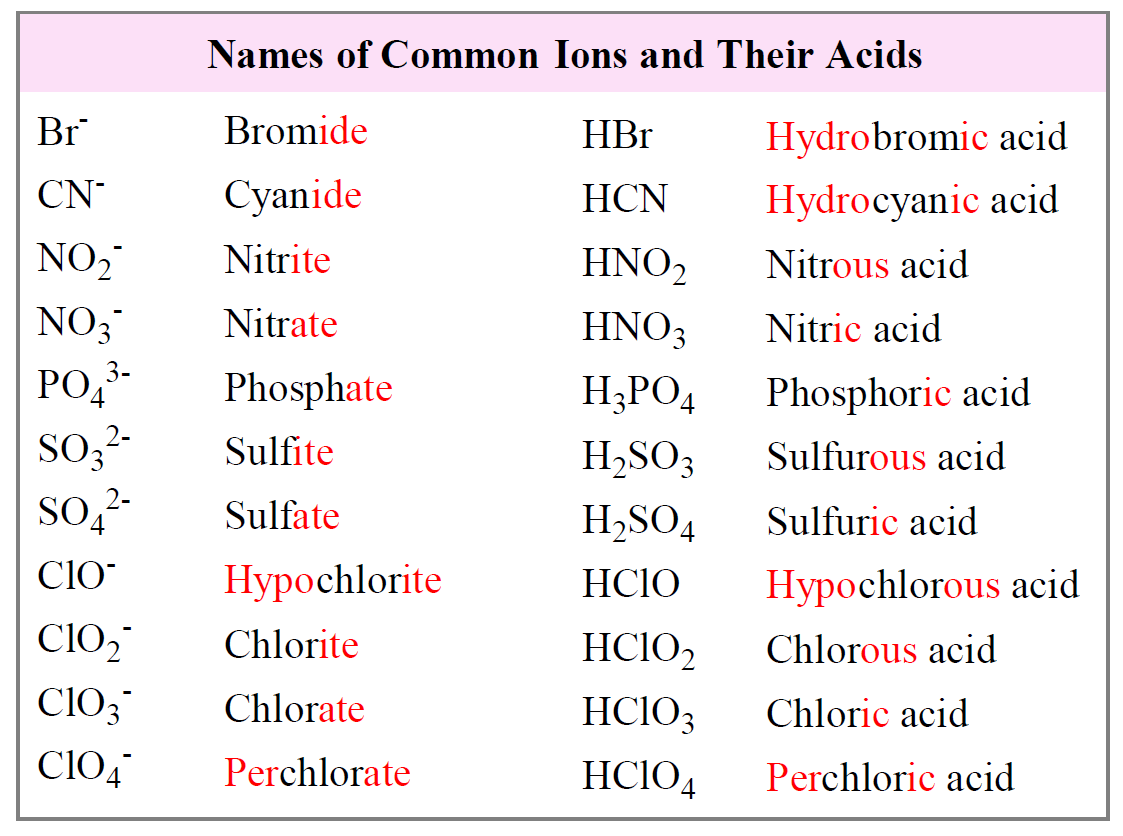
Examples of the most common types of ions and their acids are summarized in the following table:
Naming Bases
Most bases are substances that yield hydroxide ions (OH-) when dissolved in water. To name a base, add the word hydroxide to the name of the cation:
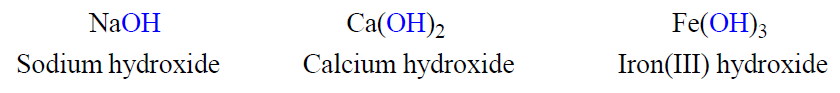
More practice examples on nomenclature and formulas can be found in this multiple-choice quiz:
Atomic Structure, Mass, and Isotopes Quiz
Nomenclature and Formulas Quiz
Check Also


Acids and ions