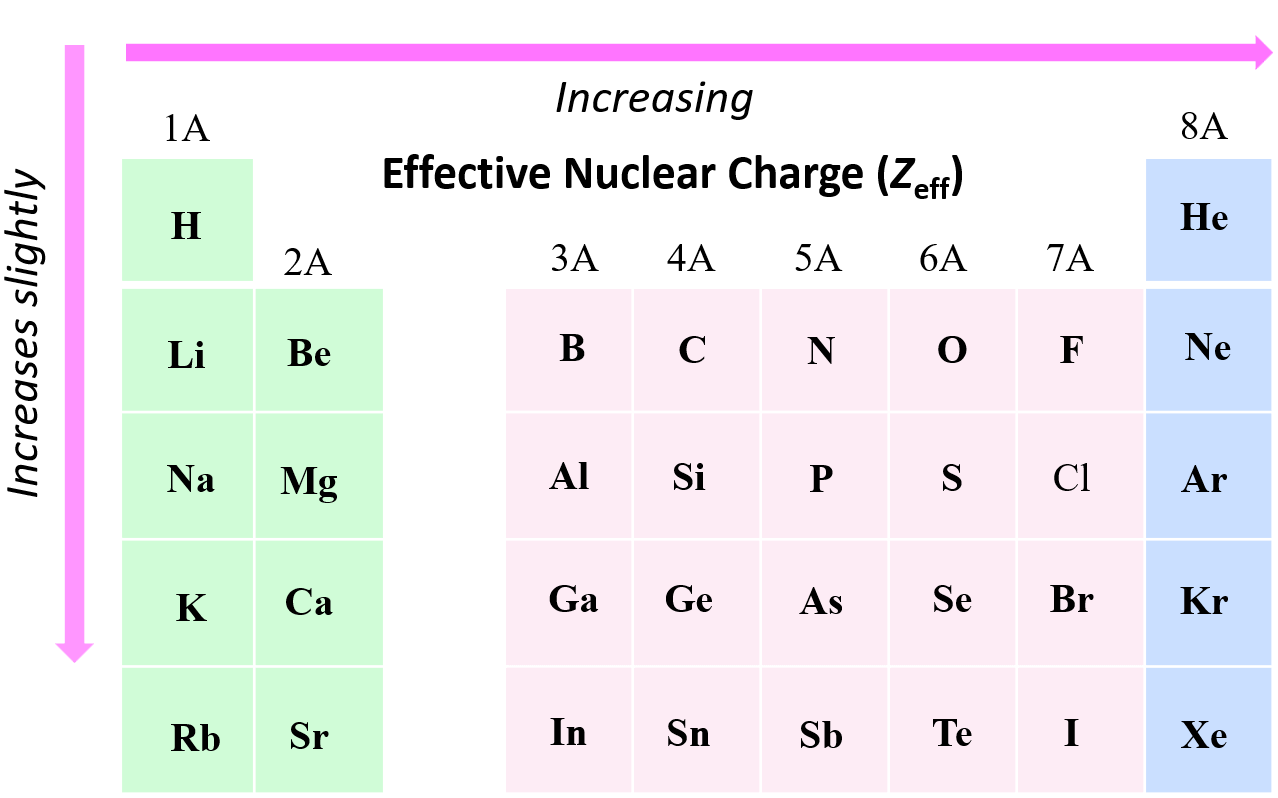
Oxidation State Practice Problems
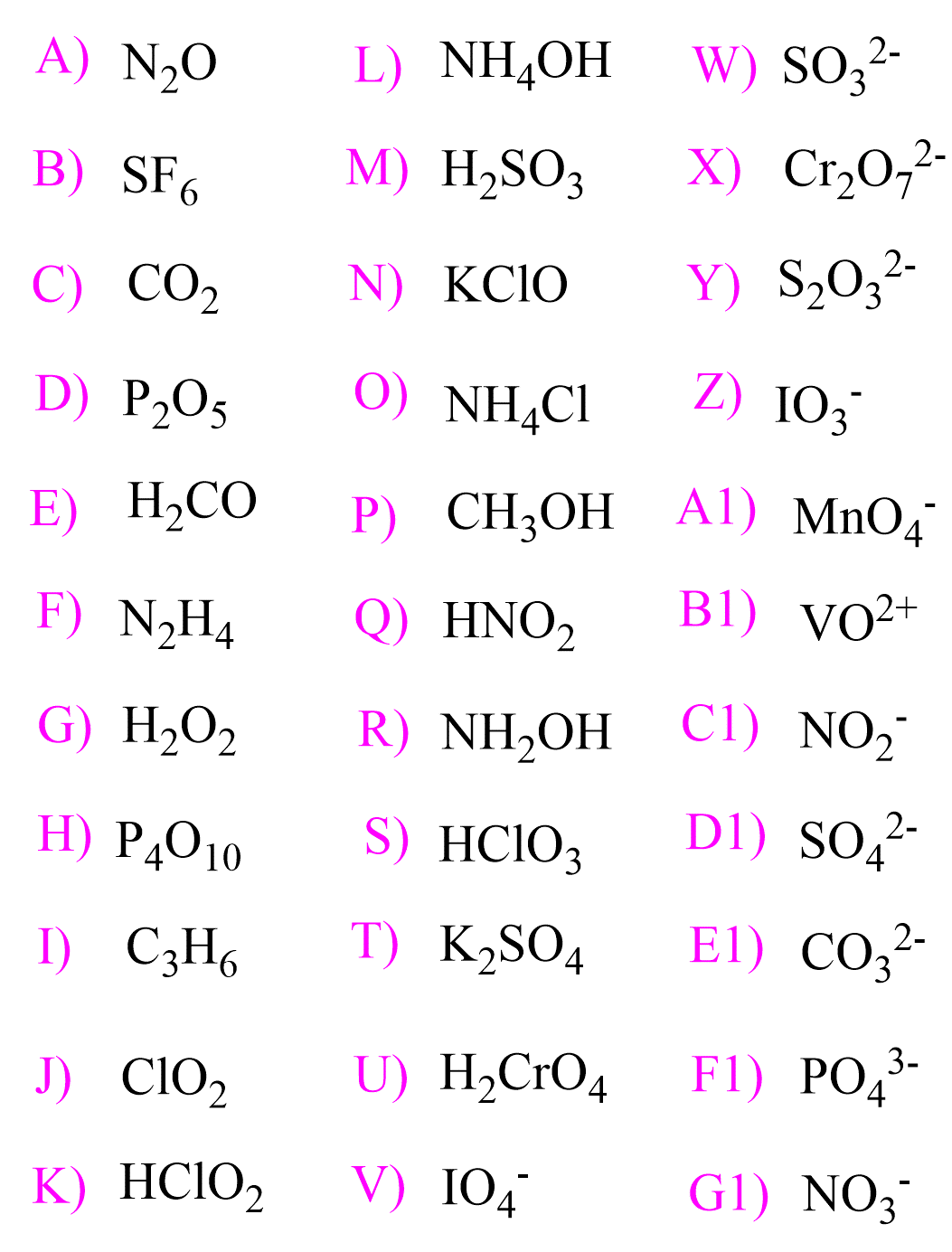
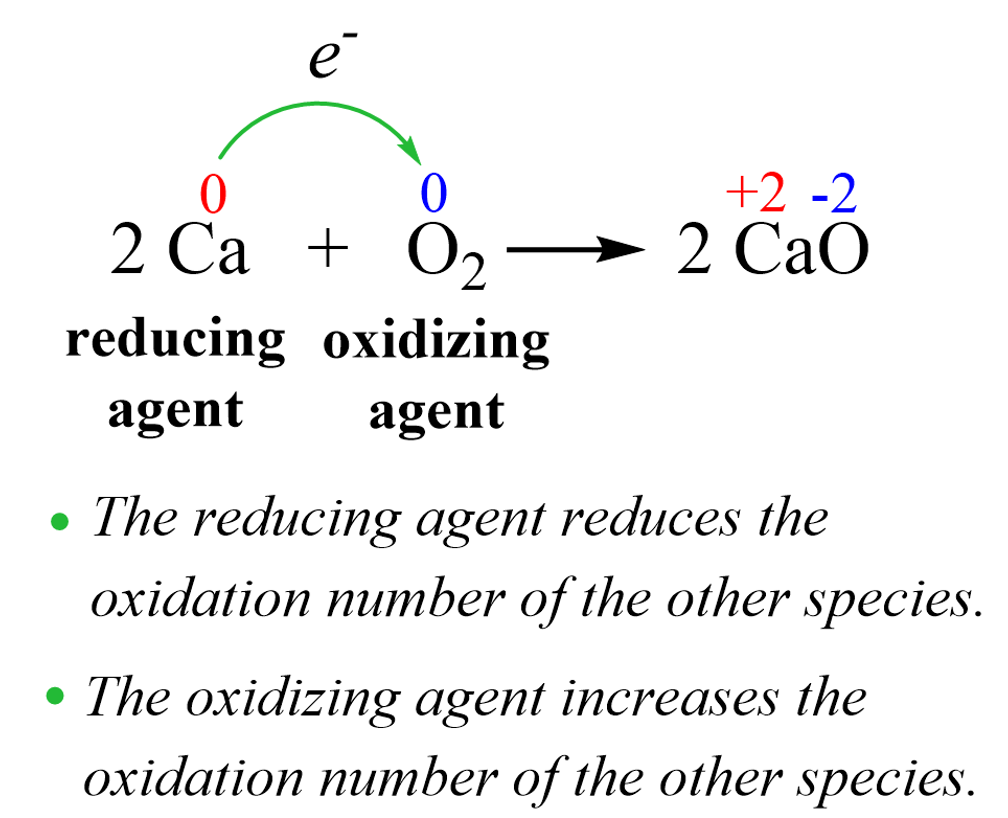
In the previous post, we talked about the oxidation state and the rules for their determination. In short, you need to know, or have the table, for the standard oxidation states, and determine the unknowns. Keep in mind, … Read more