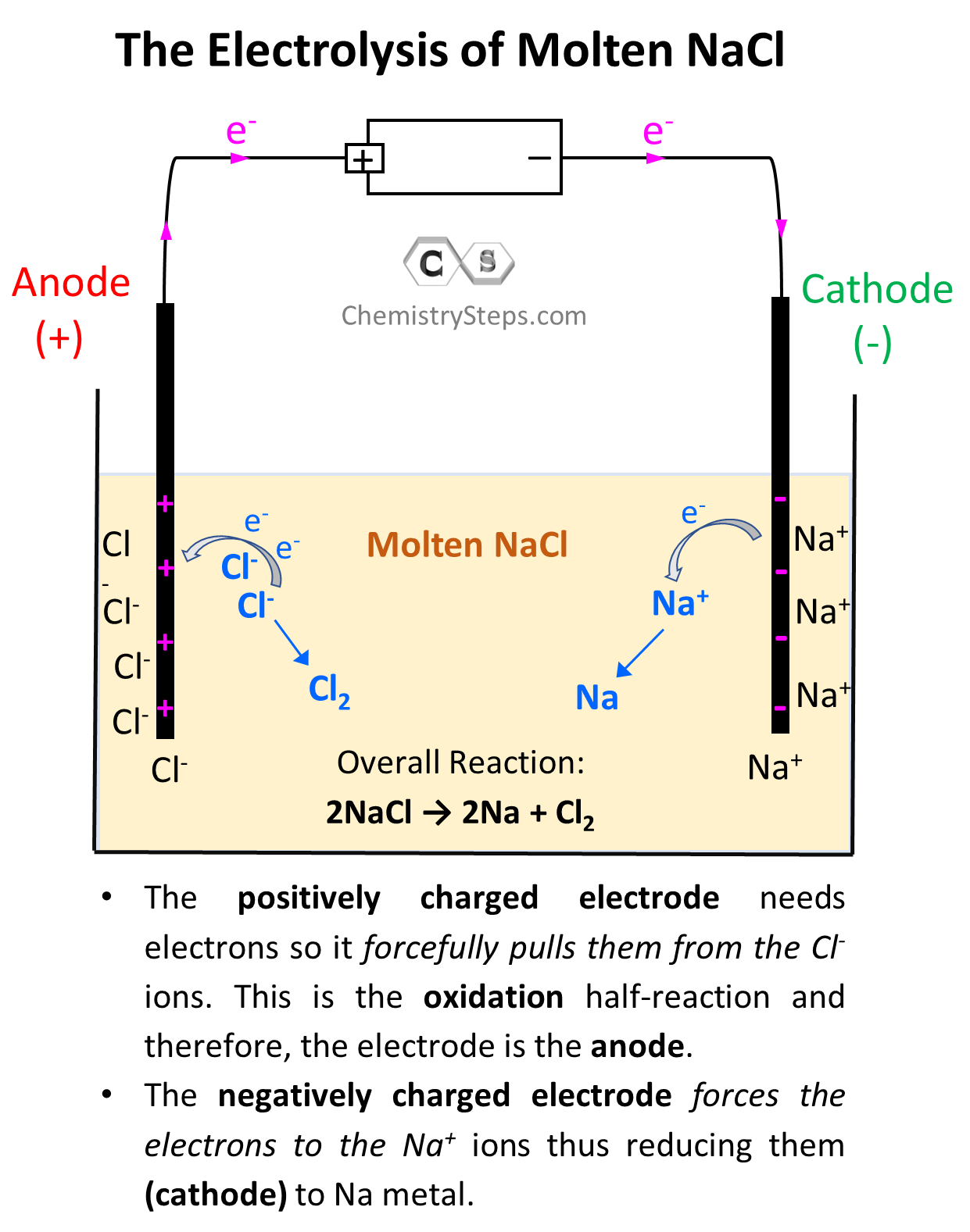
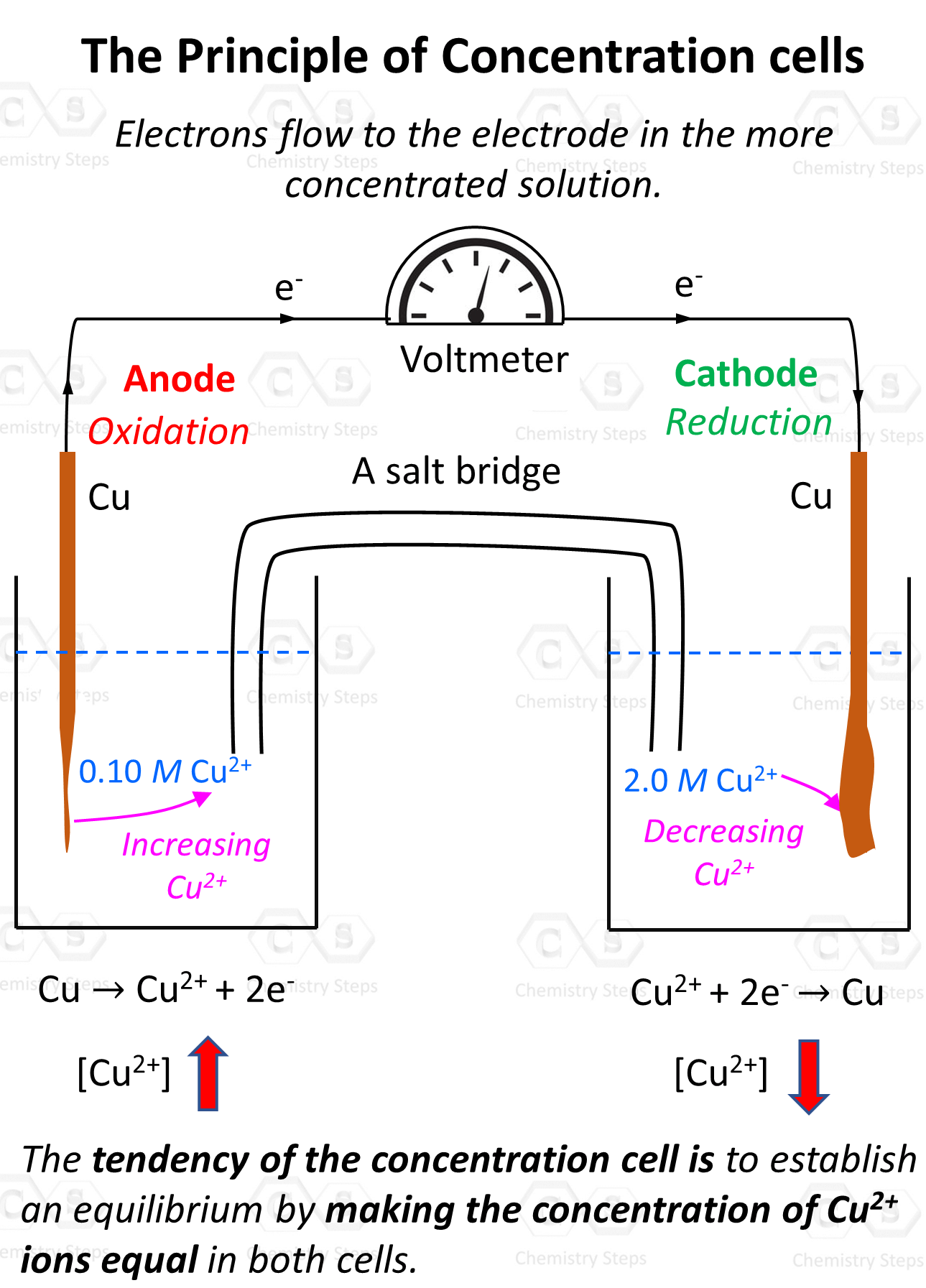
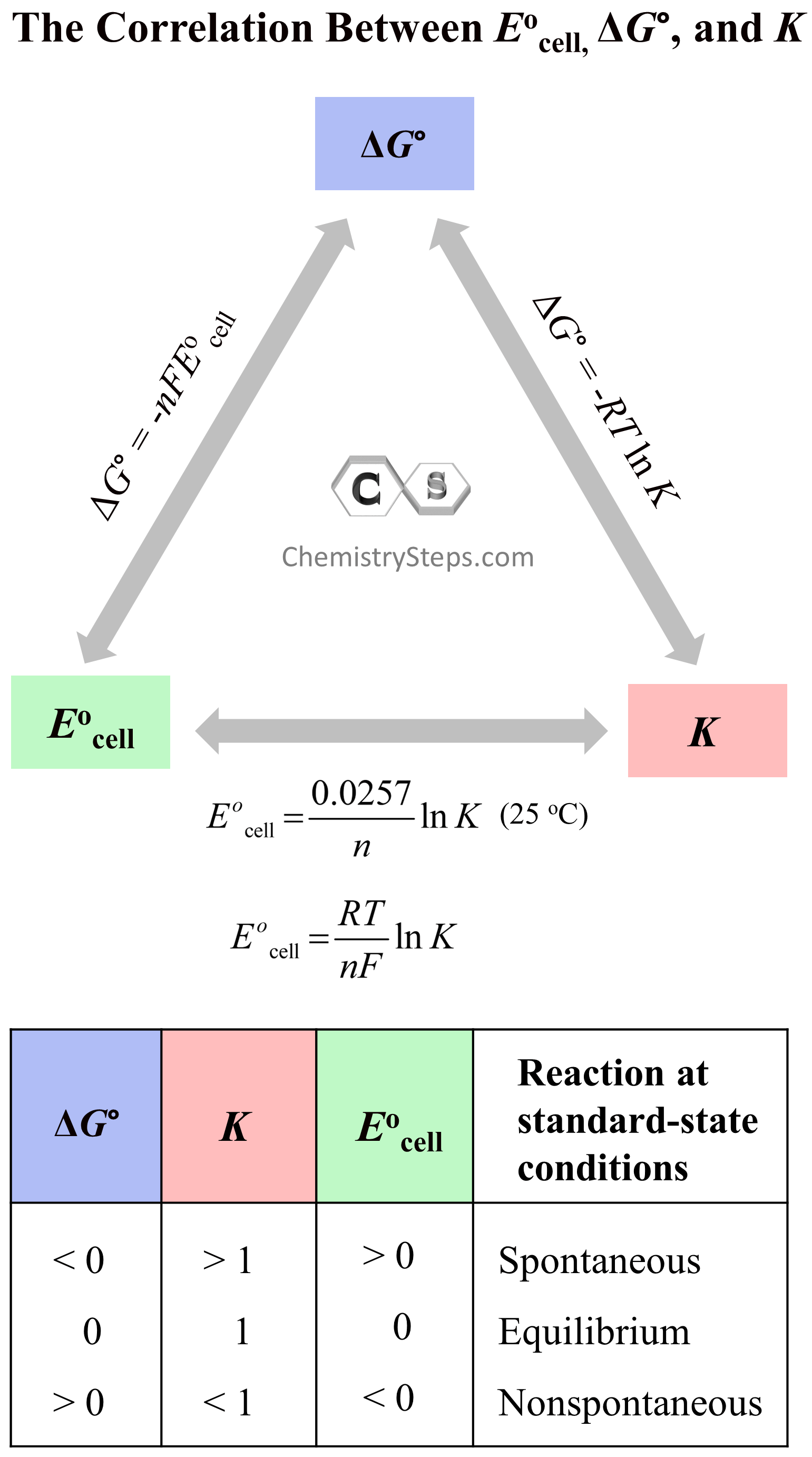
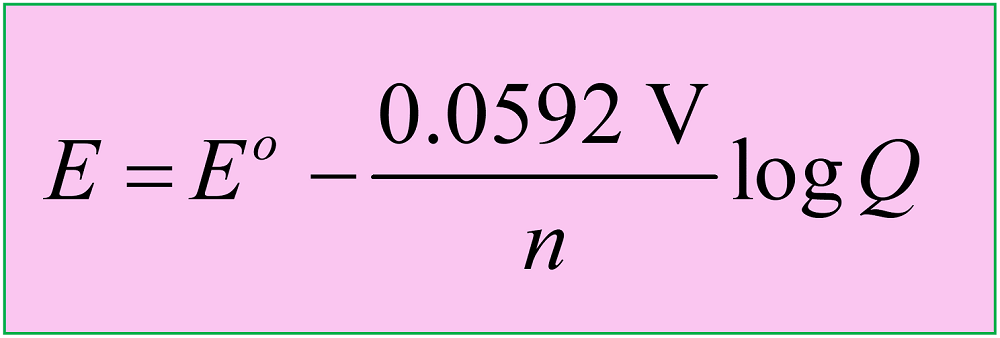Electrolytic Cells
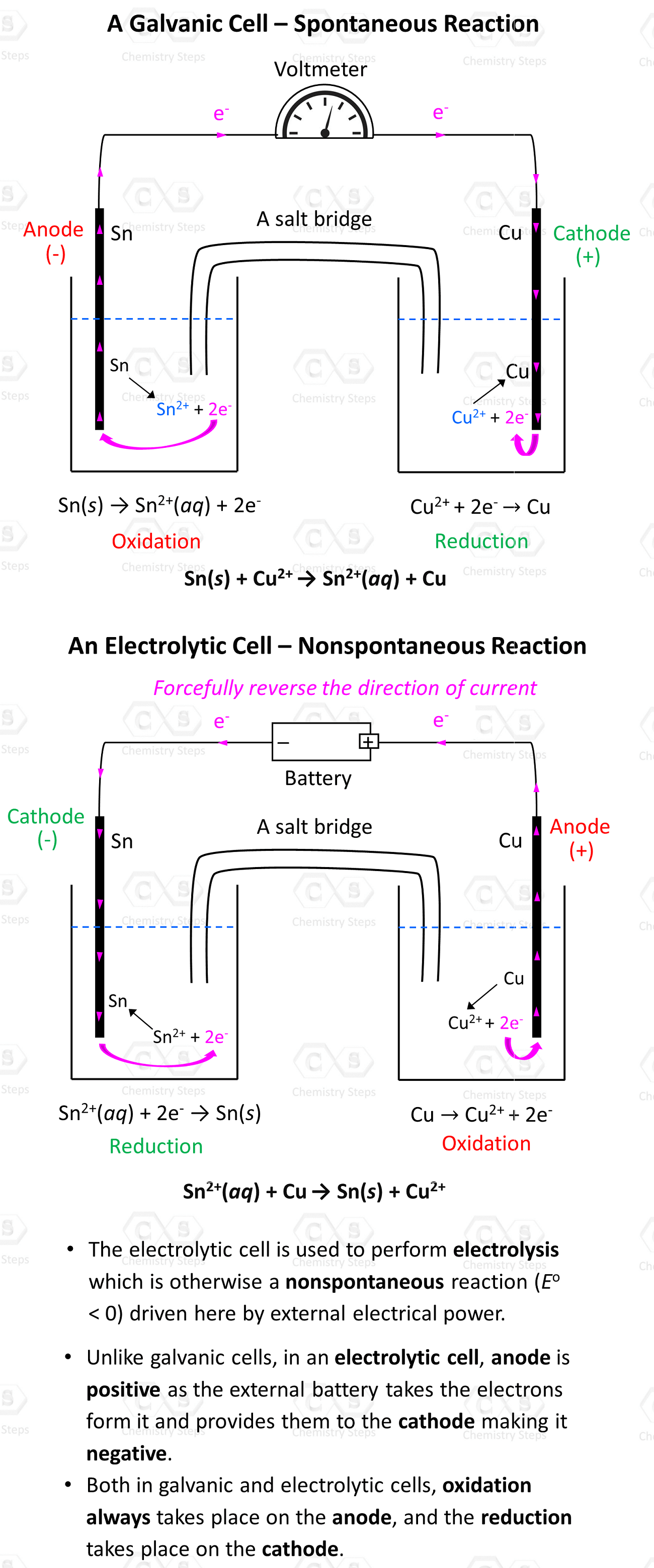
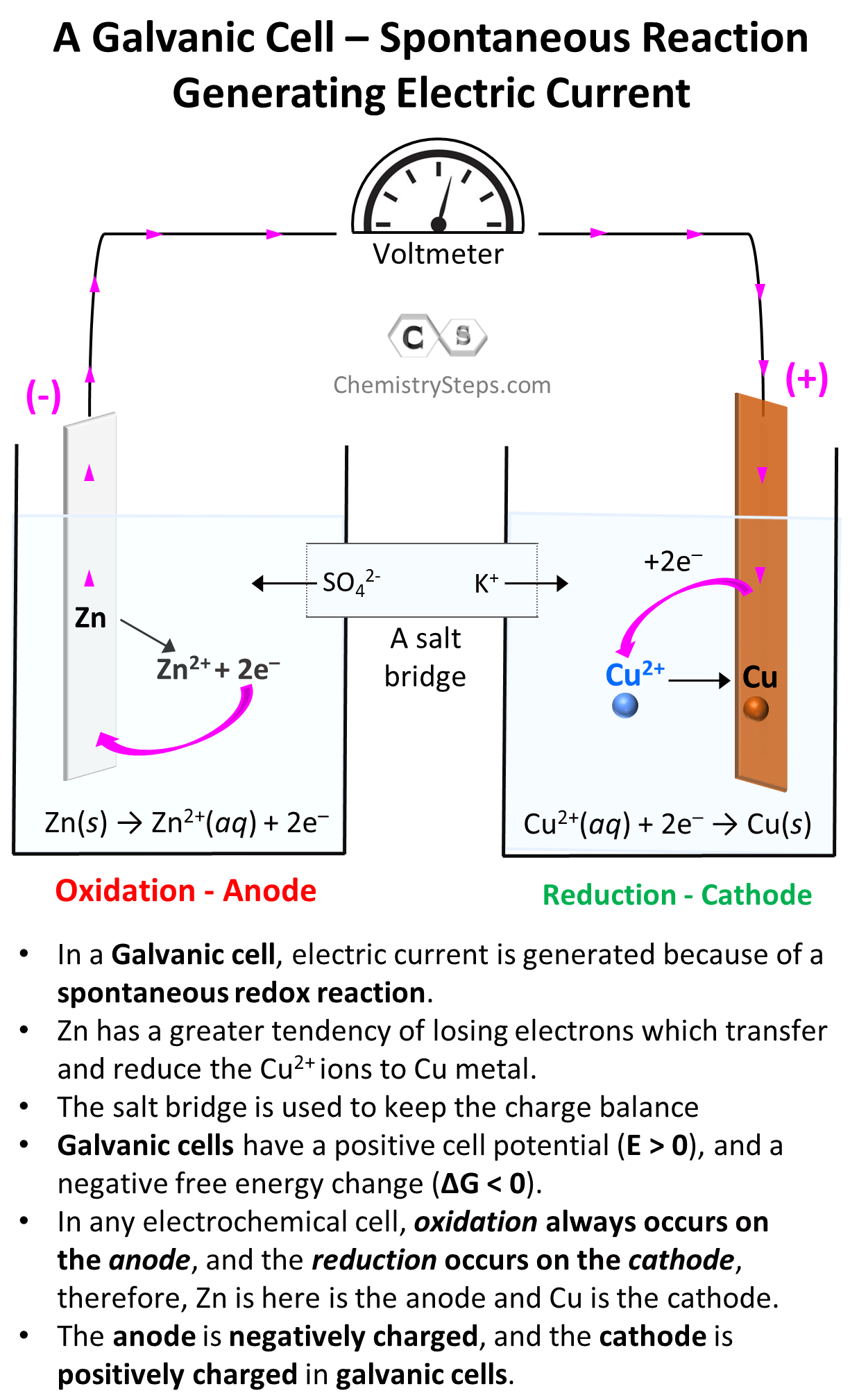
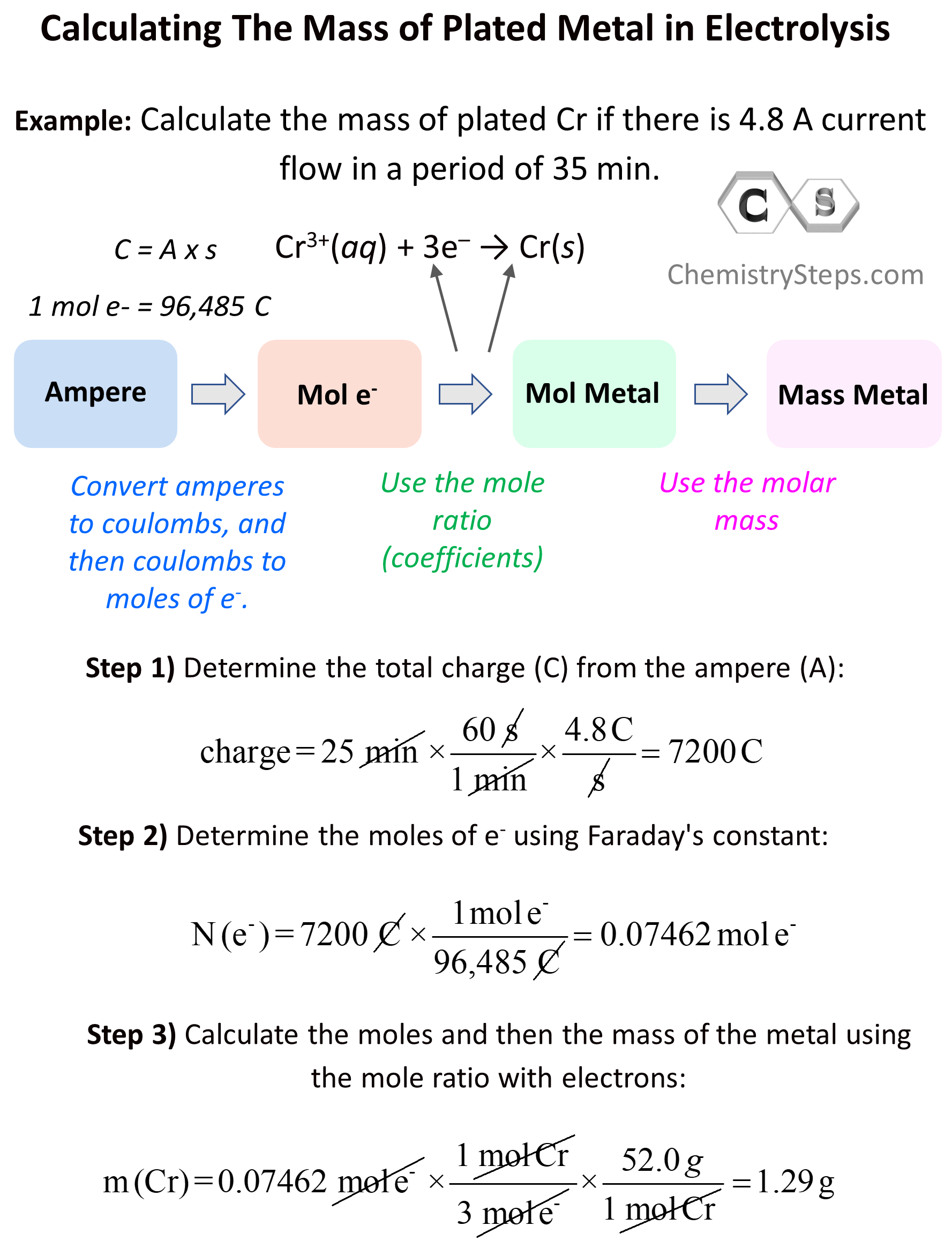
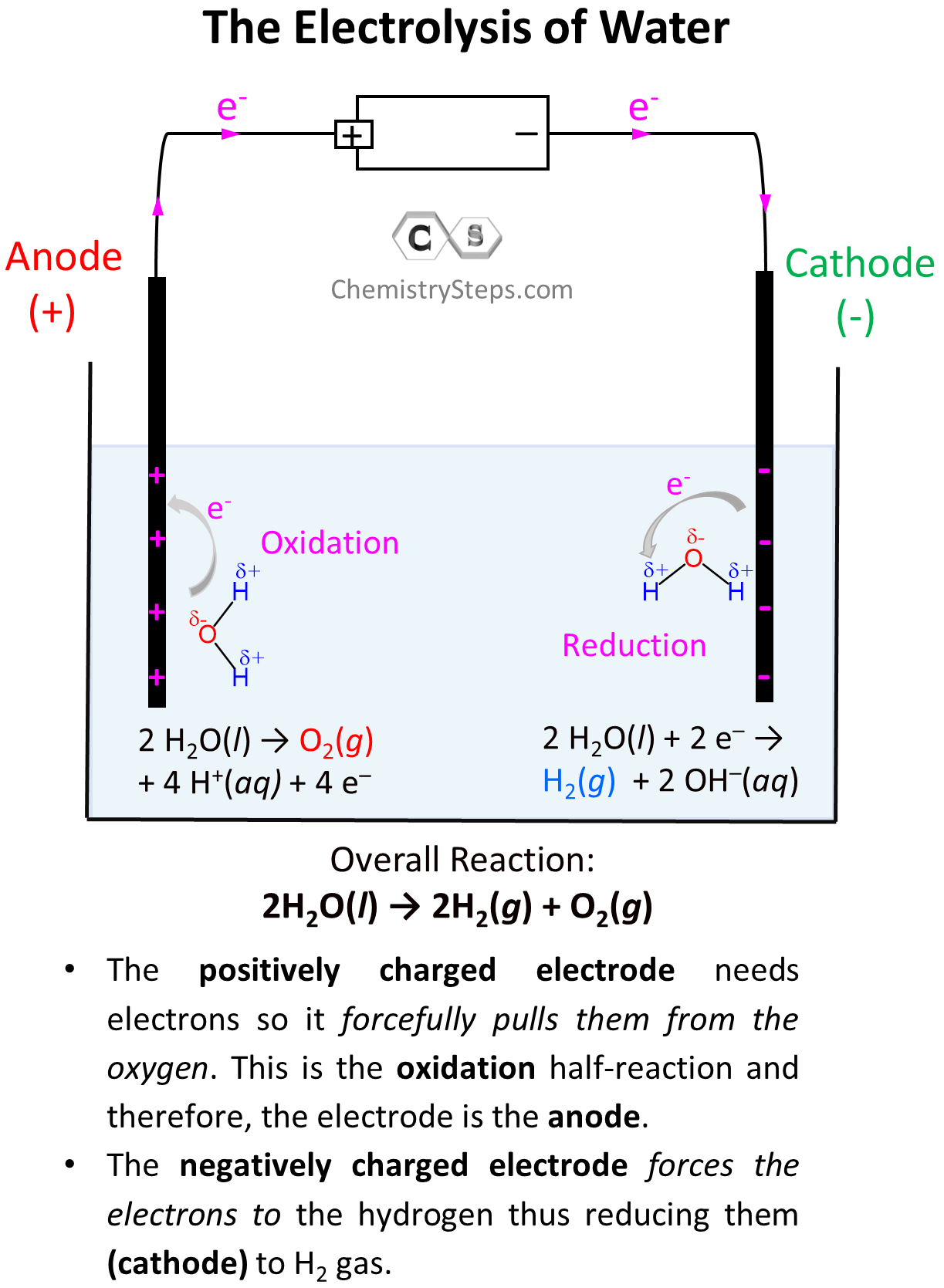
Everything we discussed so far was about Galvanic cells which generate electric current because of spontaneous redox reactions. Galvanic cells, therefore, are characterized by positive cell potential (E) and negative free energy (ΔG). The classic example for illustrating the principle … Read more