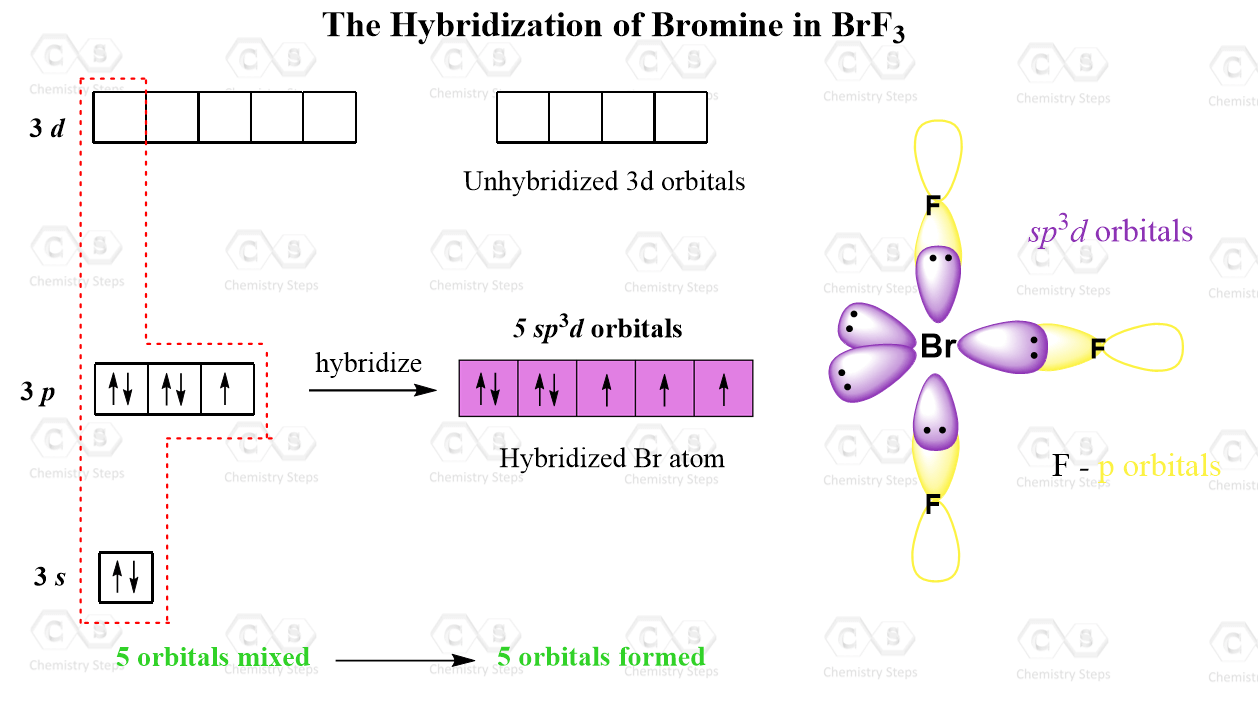
Br is the central atom:
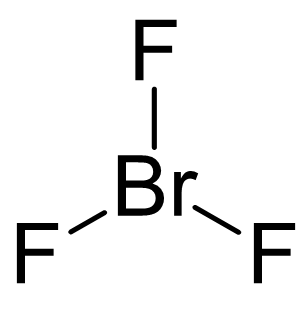
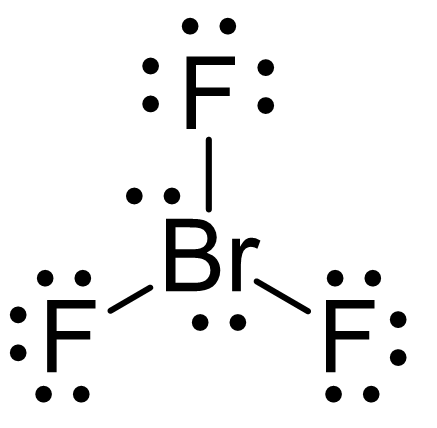
There are 7 + 3×7 = 28 electrons and 6 are taken to make three covalent bonds. Each fluorine takes 6 electrons, therefore there are 28 – (6 + 3×6) = 4 electrons left, which go on the Br as two lone pairs:
The central atom has 3 atoms and 2 lone pairs, therefore, the electron geometry is trigonal bipyramidal, while the molecular geometry is T-shaped:
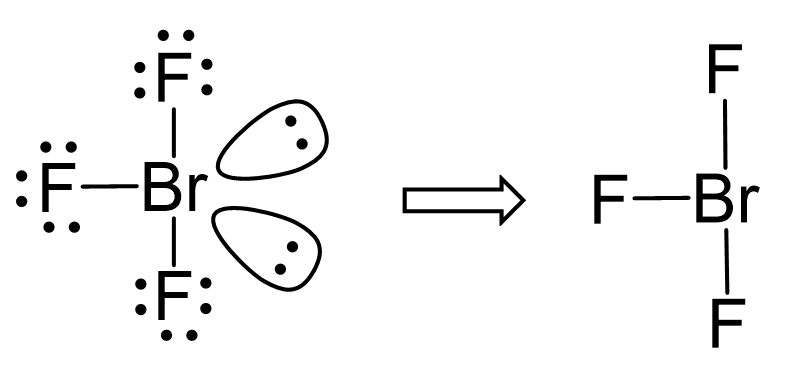
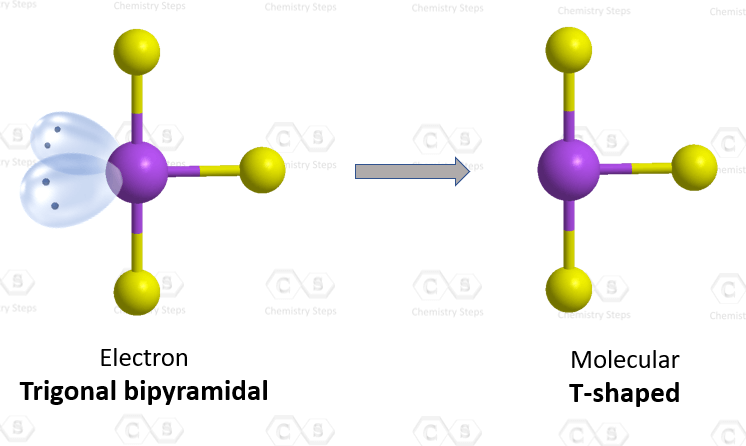
Notice that the lone pair does not go in axial positions (up or down).
There are 5 units (atoms and lone pairs) on the central atom, and to accommodate them, it needs 5 orbitals which is achieved through sp3d hybridization.
Check this 99-question multiple-choice quiz on Geometry and Hybridization:
Free
Geometry and Hybridization Quiz
Check Also
- The VSEPR Model
- VSEPR Theory Practice Problems
- Hybridization of Atomic Orbitals
- sp, sp2, sp3, sp3d, and sp3d2 Hybridization Practice Problems

