First, we need to draw the Lewis structure of IF5.
In short, these are the steps you need to follow for drawing a Lewis structure:
1. Write the correct skeletal structure for the molecule.
* Hydrogen atoms are always terminal (only one bond)
* Put more electronegative elements in terminal positions
2. Sum the valence electrons from all the atoms.
3. Use a pair of electrons to form a bond between each pair of bound atoms.
4. Add the remaining electrons to satisfy the octet for a more electronegative atom first.
5. If any atoms lack an octet, make a double or triple bond to give them an octet.
Br is the central atom, so we can draw a preliminary skeletal structure:
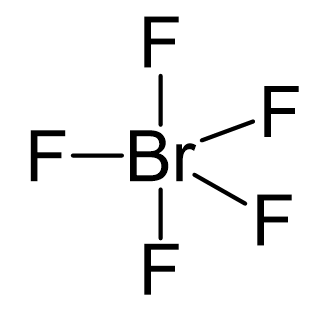
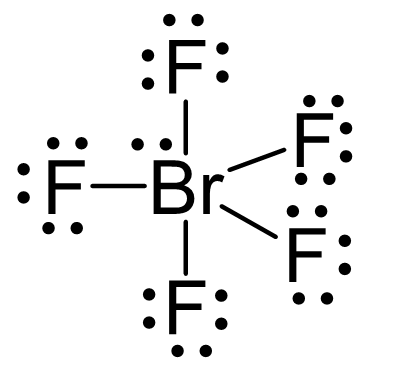
There are 5×7 + 7 = 42 electrons, out of which, 10 are used to make 5 covalent bonds. The remaining 30 are divided between the five fluorine atoms, each taking 6 electrons as 3 lone pairs, and Br takes the last pair of electrons:
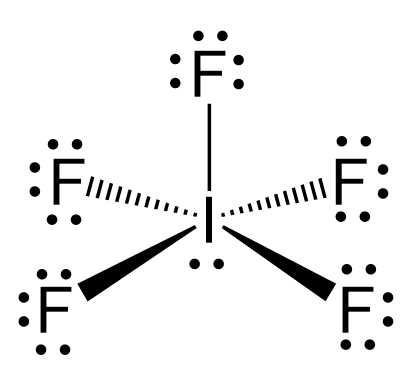
The hybridization of iodine in IF5 is the same as bromine in BrF5, and we have seen in problem 23 that there are 5 atoms and one lone pair on the central atom, therefore, the electron geometry is octahedral, while the molecular geometry is square pyramidal:
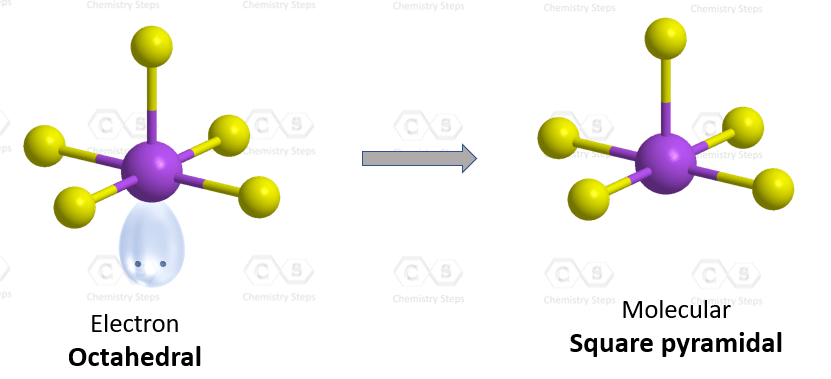
Steric number six indicates sp3d2 hybridization.
Check this 99-question multiple-choice quiz on Geometry and Hybridization:
Geometry and Hybridization Quiz
Check Also

