Sulfur is the central atom, so we can draw a preliminary skeletal structure:
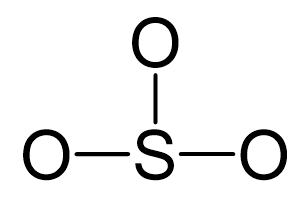
There are 3×6 + 6 = 24 electrons, and 6 of them are used to make 3 bonds. Three oxygens take 6 lone pairs and make an additional bond with the sulfur.
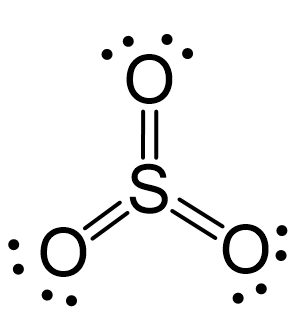
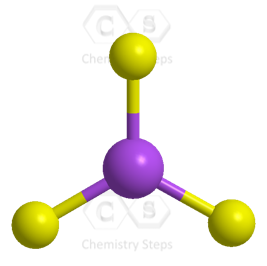
All the electrons have been used, and the steric number of the central atom is 3 with no lone pairs. Therefore, both geometries are trigonal planar.
The steric number (the sum of the number of the atoms and lone pairs) of the carbon is 3 which corresponds to sp2-hybridization.
Check Also
