Phosphorous is the central atom, so we can draw a preliminary skeletal structure:
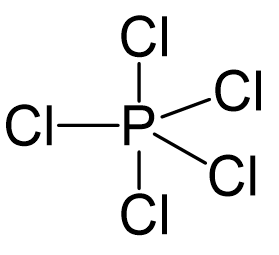
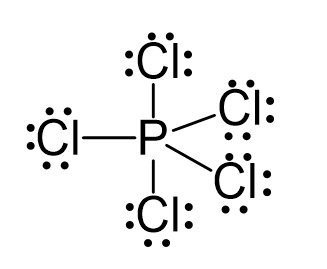
There are 5×7 + 5 = 40 electrons, out of which, 10 are used to make 5 covalent bonds. All the remaining 30 are divided between the five chlorine atoms, each taking 6 electrons as 3 lone pairs:
There are 5 atoms and no lone pairs on the central atom, therefore, both geometries are trigonal bipyramidal:
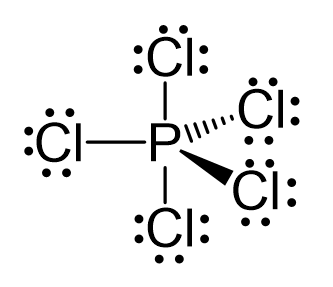
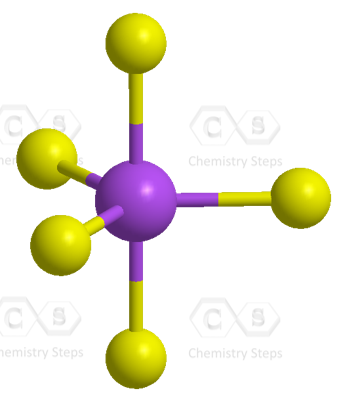
The steric number is 5, and therefore, there should be 5 orbitals accommodating the bonding electrons. This is achieved through sp3d hybridization:
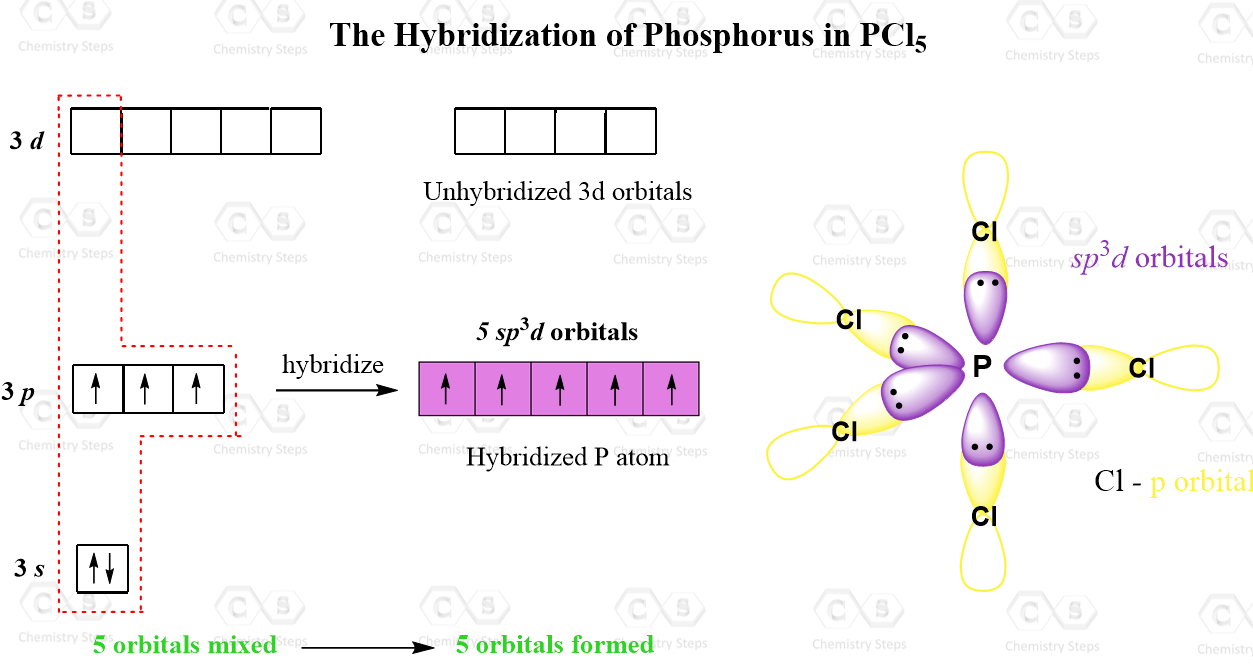
Even though there is a constant debate about whether the d orbitals are involved in these types of hybridizations, this answer is usually accepted in undergraduate general chemistry courses. Ultimately, it is on your professor, as they are the authority in the class.
More practice examples on geometry and hybridization in this multiple-choice quiz:
Geometry and Hybridization Quiz
Check Also

