P is the central atom and there are 5 + 3×7 = 26 electrons. Each chlorine taking three lone pairs leaves the nitrogen with one lone pair:
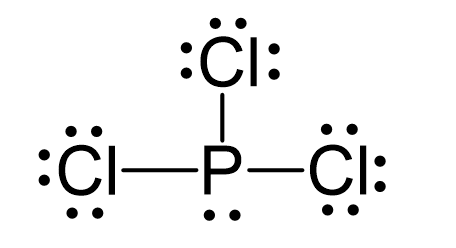
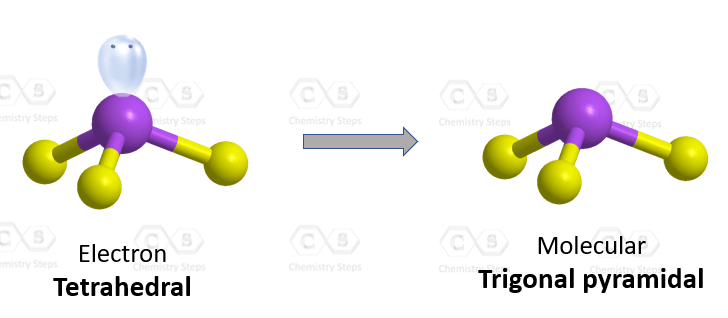
The central atom has 3 atoms and a lone pair (SN = 4). Therefore, the electron geometry is tetrahedral while the molecular geometry is trigonal pyramidal:
Steric number 4 corresponds to sp3-hybridization where the idealized bond angles are 109.5o. Because the groups on the central atom are not identical, the bond angles differ from the idealized values.
Check Also
