In the previous post, we talked about naming monatomic (monoatomic) and polyatomic ions. Once you know the names of the common ions, it is not difficult to name ionic compounds as we only need to put the names of the cation and the anion together.
Naming Binary Ionic Compounds
Binary ionic compounds are made of only two elements. To name a binary ionic compound, identify the ions and list the cation first followed by the anion.
Remember, group 1 and 2 cations inherit the name of the metal they are derived from, and monoatomic anions take the ending –ide.
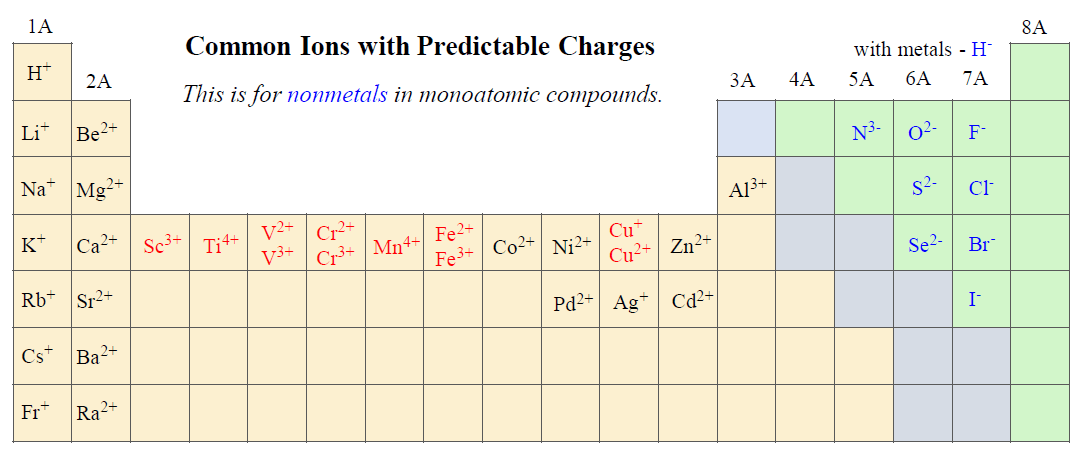
For example,
K2S – Potassium sulfide, CaBr2 – Calcium bromide, AlCl3 – Aluminum chloride, MgO – Magnesium oxide
If the compound contains a cation of a transition metal with two possible charges, then a Roman numeral or the corresponding suffix is used to indicate its charge.
The suffix for the cation with a higher charge is “ic” and for the lower charge is “ous”.
The names and formulas of metals forming different cations are summarized in the table below:
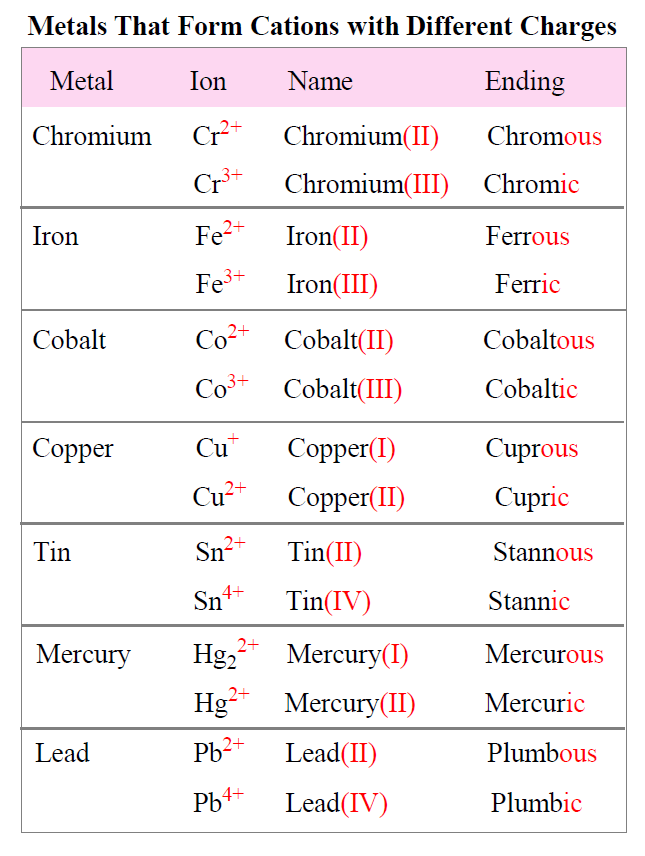
For example,
FeCl3 – Iron(III) chloride or Ferric chloride
FeCl2 – Iron(II) chloride or Ferrous chloride
Naming Compounds with Polyatomic Ions
The same principle of listing the names of the cation followed by the name of the anion applies to naming ionic compounds with polyatomic ions.
Here is the table with the names and formulas of polyatomic ions:
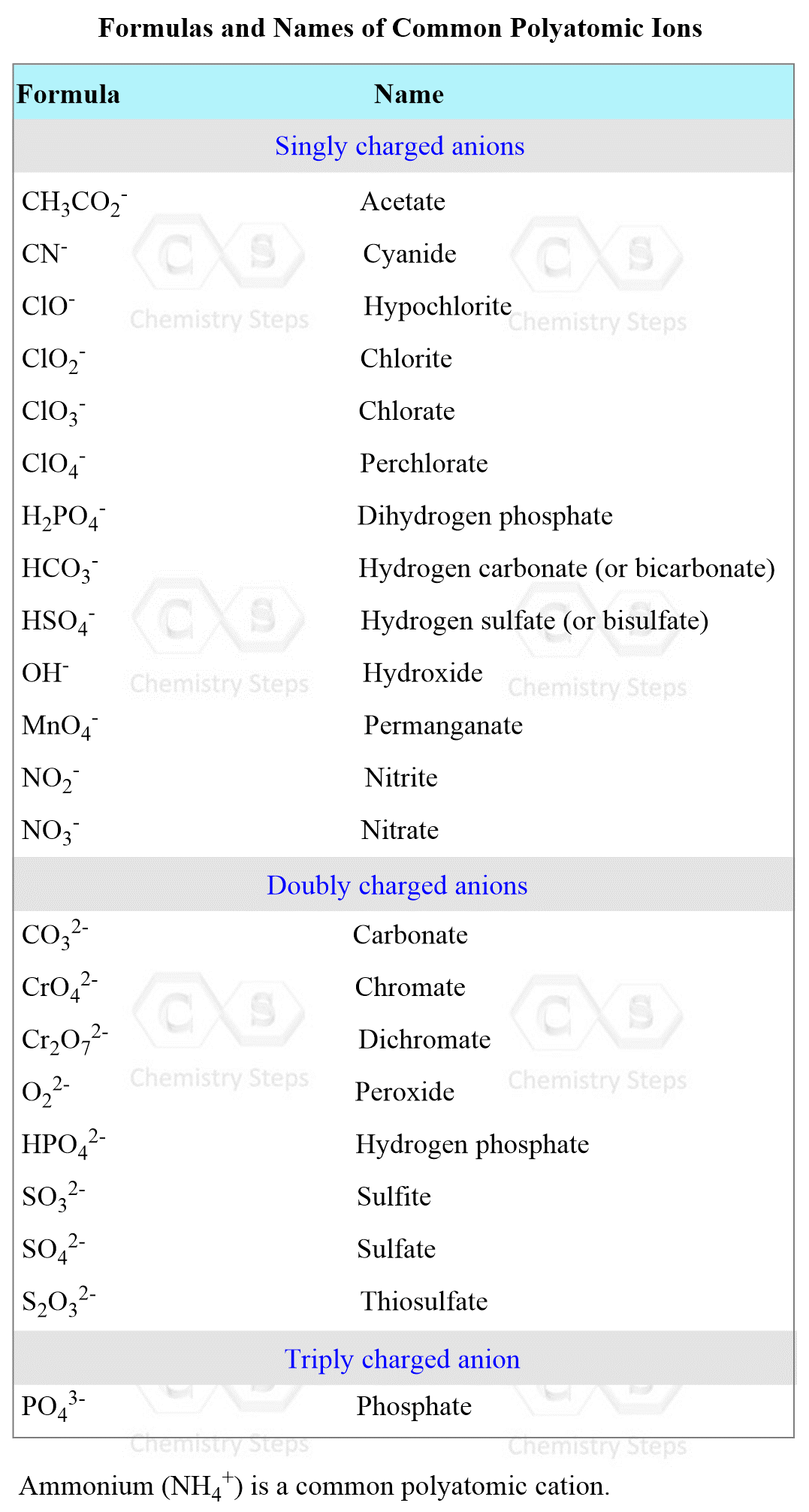
For example,
BaSO3 – Barium sulfite, Na2CrO4 – Sodium chromate, KClO4 – Potassium perchlorate
Examples of compounds containing a transition metal with two possible charges:
CuNO3 – Copper(I) nitrate or Cuprous nitrate
Cu(NO3)2 – Copper(II) nitrate or Cupric nitrate
Don’t forget to add the prefix hypo– (meaning “less than”) and per– ( “more than”) for oxyanions with more than two formulas.
For example,
Ba(ClO)2 – Barium hypochlorite
Ba(ClO2)2 – Barium chlorite
Ba(ClO3)2 – Barium chlorate
Ba(ClO4)2 – Barium perchlorate
Naming Hydrates
Hydrates contain a specific number of water molecules attached to them. The number of water molecules is represented with a dot and the Greek prefixes:
CuSO4 5H2O copper(II) sulfate pentahydrate
MgSO4 7H2O – magnesium sulfate heptahydrate
Check Also
- Subatomic particles and Isotopes
- Naming Monatomic and Polyatomic Ions
- Naming Ionic Compounds
- Naming Covalent Compounds
- Naming Acids and Bases
- Atomic and Molecular Masses
- The Mole and Molar Mass
- Molar Calculations
- Percent Composition and Empirical Formula

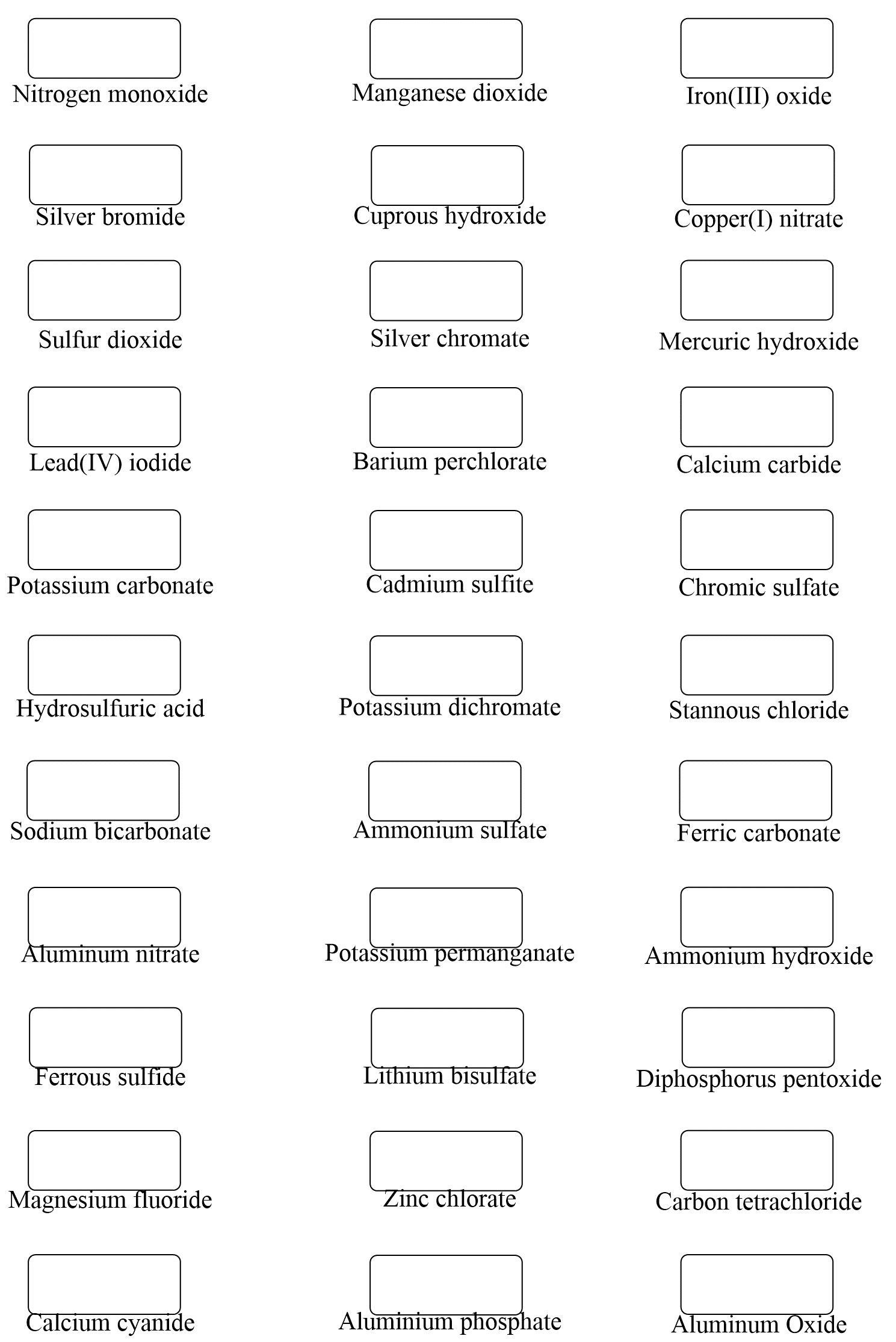
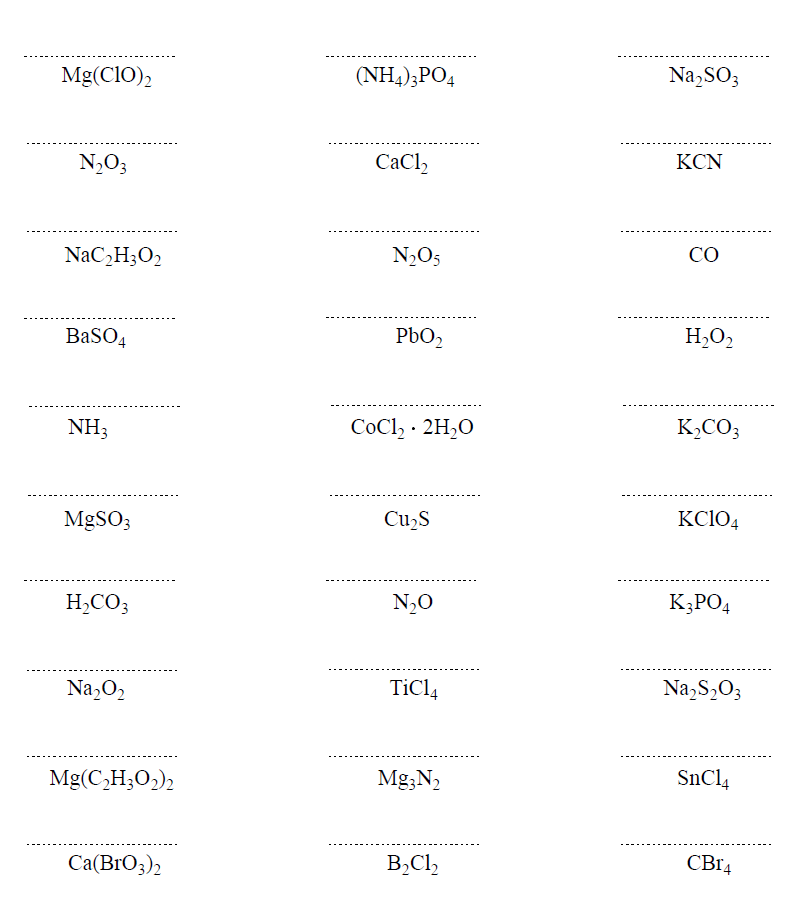
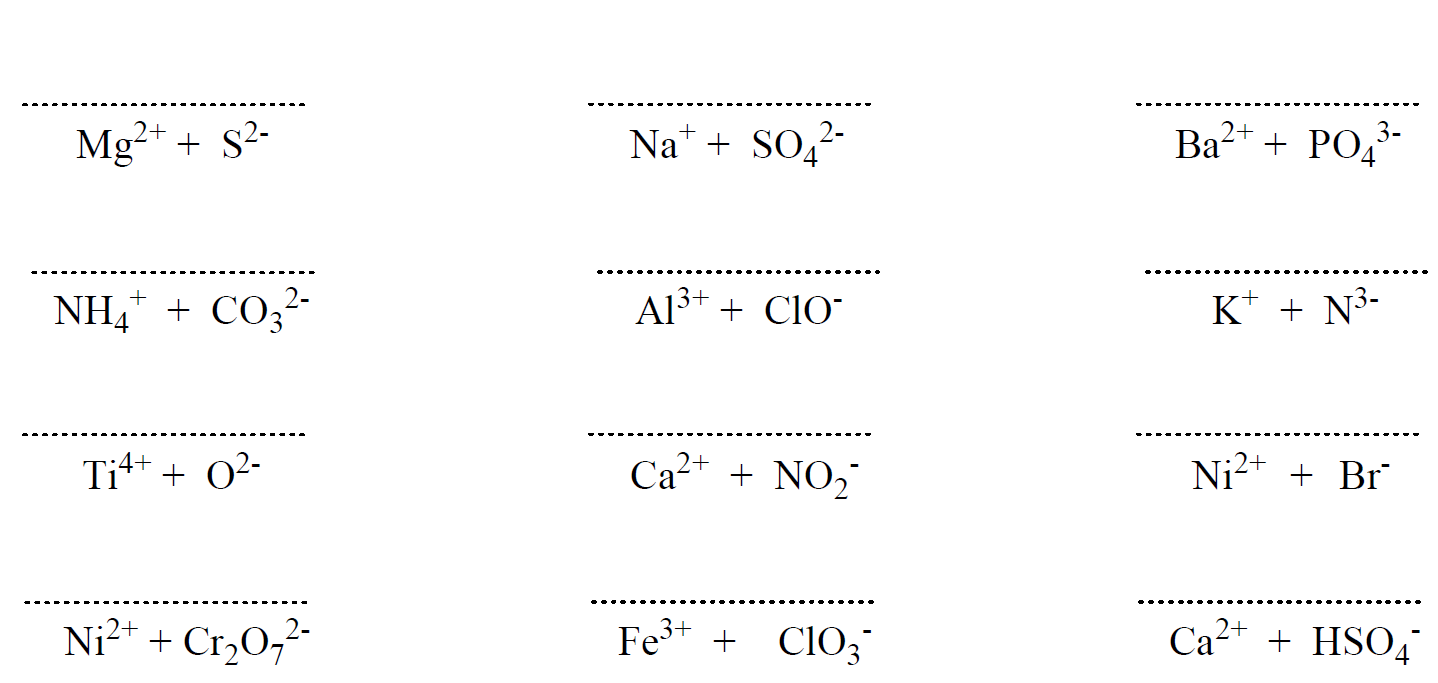
Hi,
It’s not a huge mistake, but I realized there’s one that says Ferric Cyanide (all the way at the bottom of problem set 1) while the answer key says Calcium Cyanide. I don’t think it matters that much because both Ferric and Calcium have a charge of +2 🙂
Uh-oh… I will fix that but don’t forget ferric is for the higher charge of iron which is +3. The ion with a lower charge, Fe2+ is the ferrous. Thanks again!