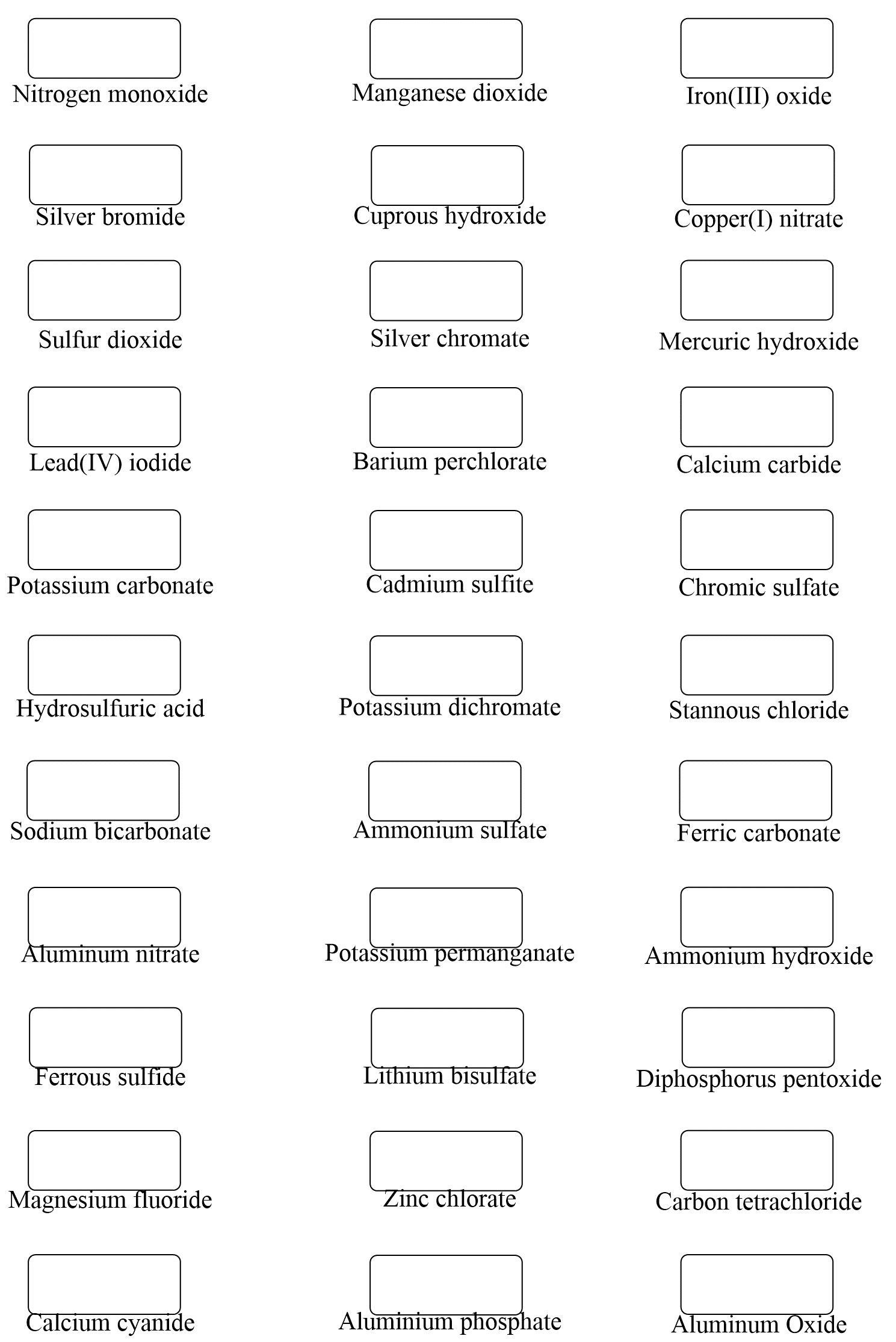
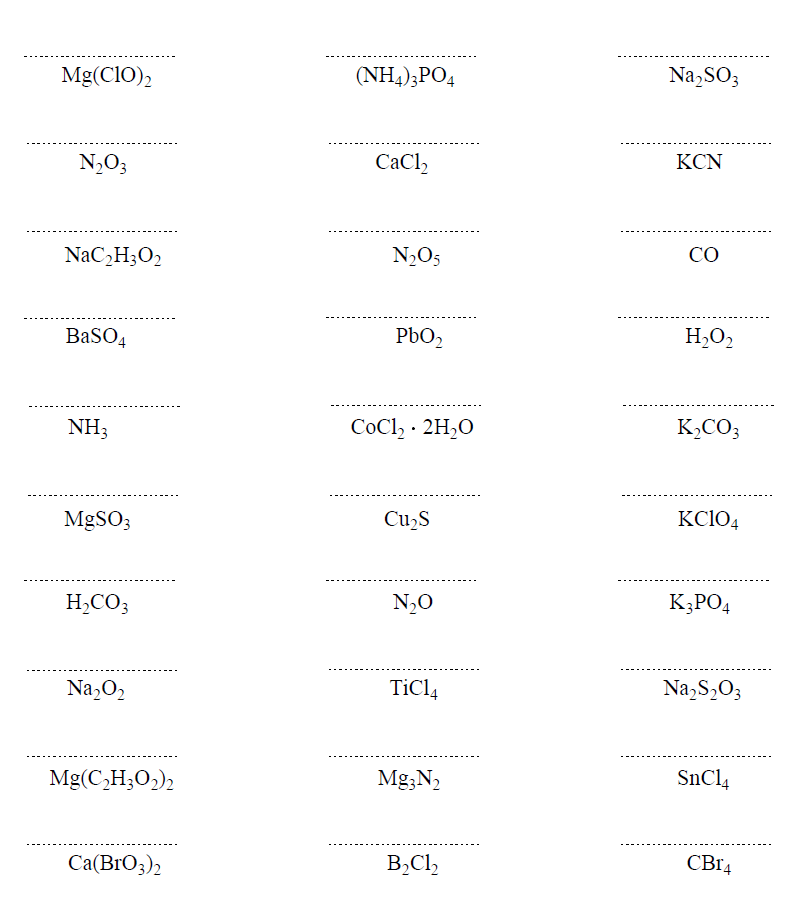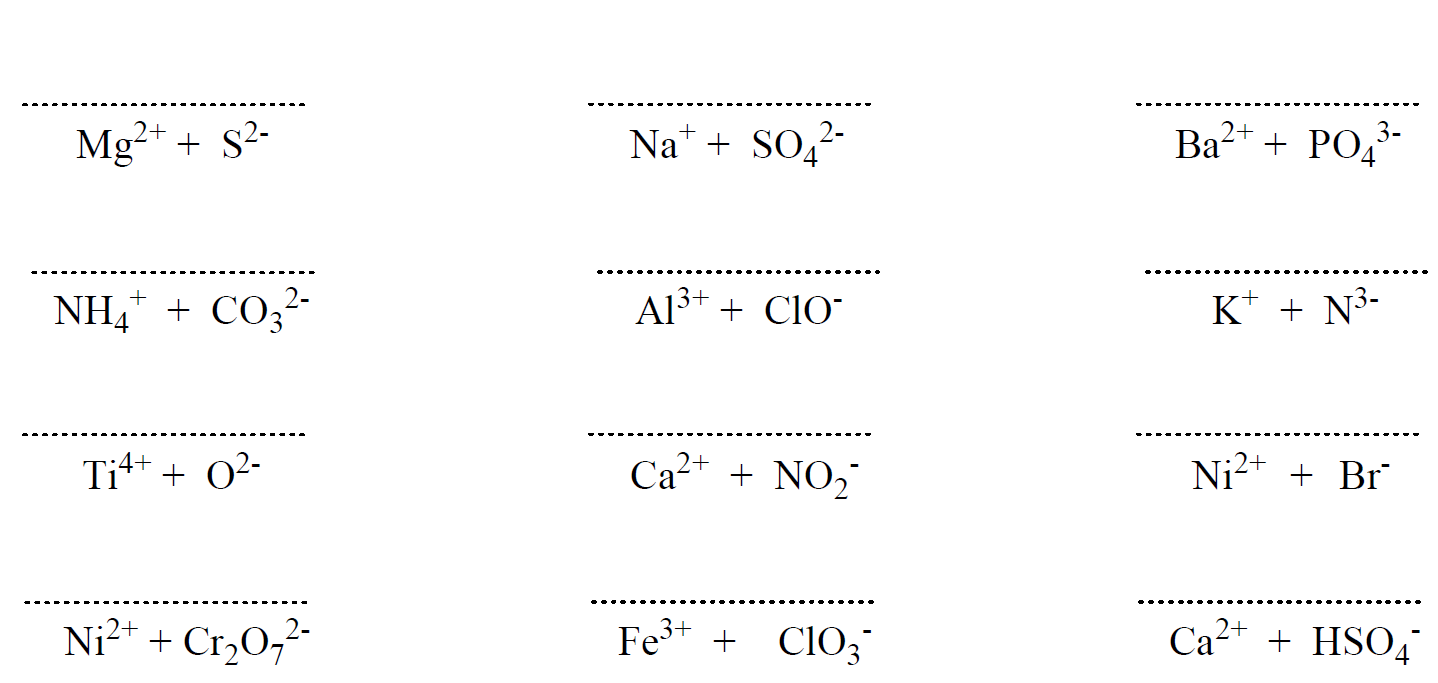Naming molecular compounds is similar to naming ionic compounds in that we treat the first element as if it is a cation, while the second element is an anion. This means the name of the first element is what it is in the periodic table, while for the second element, we change the ending to -ide.
A reminder that a molecule is a group of two or more atoms held together by covalent bonds. Covalent bonds are usually formed between nonmetals. For example, Cl2, CH4, NH3, CO2.
One difference with naming ionic compounds though is that we add prefixes to denote the number of atoms in the molecule.
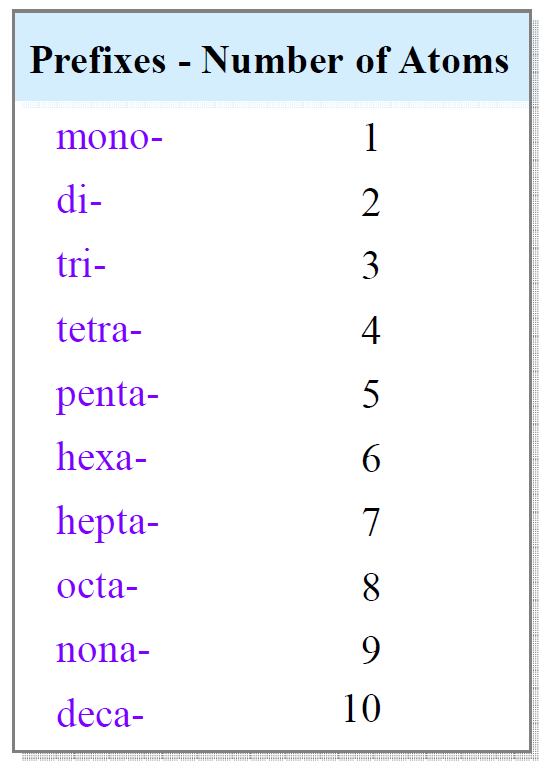
For example,
SF6 – Sulfur Hexafluoride
NCl3 – Nitrogen trichloride
CO2 – Carbon dioxide
N2O3 – Dinitrogen trioxide
A few exceptions to using the prefixes:
- The prefix mono- is never used for naming the first element. For example, we don’t say monocrbon monoxide, but rather carbon monoxide.
- Prefixes are usually not used for compounds containing a metal. Al2S3 – Aluminum sulfide, not dialuminum trisulfide, although this can also be seen sometimes.
- Molecular compounds containing hydrogen are also exceptions to using these prefixes are often used by common nomenclature.
For example,
NaH – sodium hydride and not sodium monohydride
B2H6 – diborane
CH4 – methane
SiH4 – silane
Check Also
- Subatomic particles and Isotopes
- Naming Monatomic and Polyatomic Ions
- Naming Ionic Compounds
- Naming Covalent Compounds
- Naming Acids and Bases
- Atomic and Molecular Masses
- The Mole and Molar Mass
- Molar Calculations
- Percent Composition and Empirical Formula