Carbon is the central atom, so we can draw a skeletal structure first:
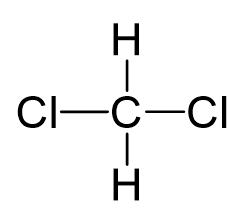
There are 4 + 2 + 2×7 = 20 electrons, and 8 have been used to make four bonds. The remaining 12 go on the two chlorines as lone pairs:
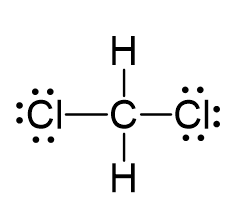
The central atom has four atoms and no lone pair, therefore, both the electron and molecular geometries are tetrahedral:
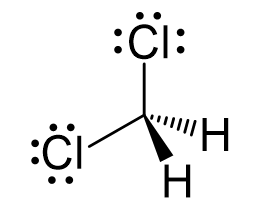
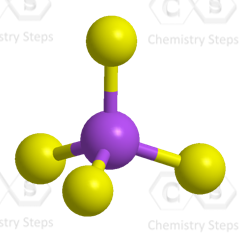
Steric number 4 corresponds to sp3-hybridization where the idealized bond angles are 109.5o.
More practice examples on geometry and hybridization in this multiple-choice quiz:
Free
Geometry and Hybridization Quiz
Check Also

