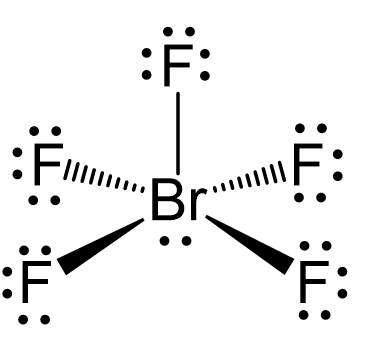
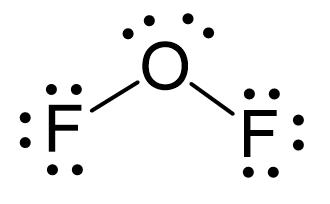
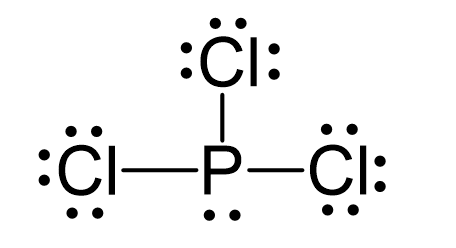
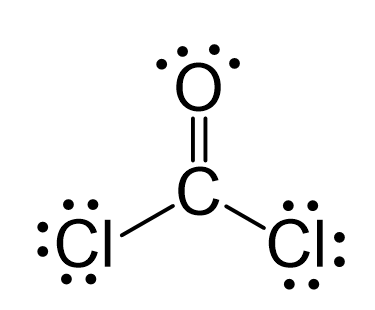
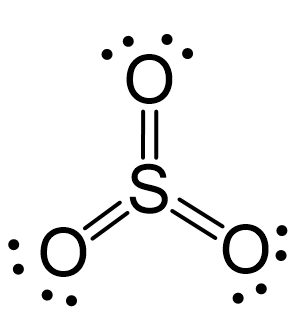
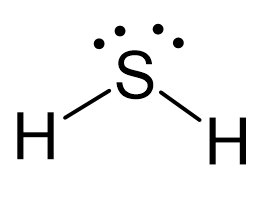
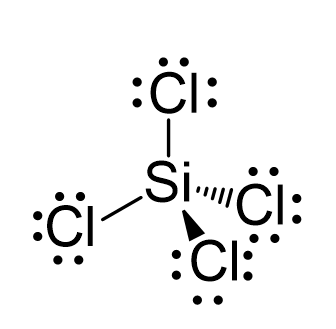
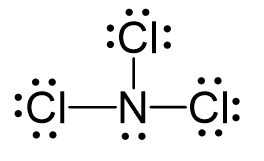
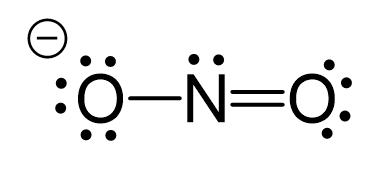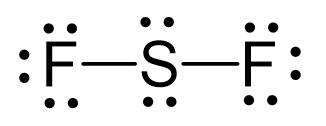N2O Geometry and Hybridization
Oxygen is more electronegative, therefore, it goes in a terminal position: There are 2×5 + 6 = 16 valence electrons and four are already taken to make two bonds. The remaining 12 are distributed between the oxygen and … Read more