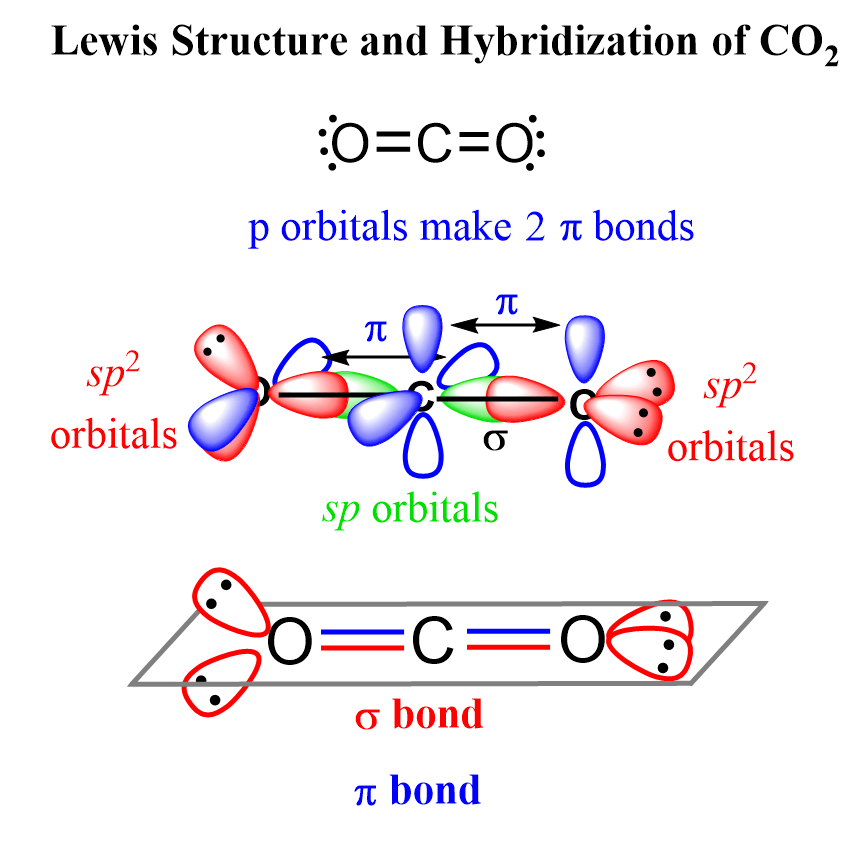
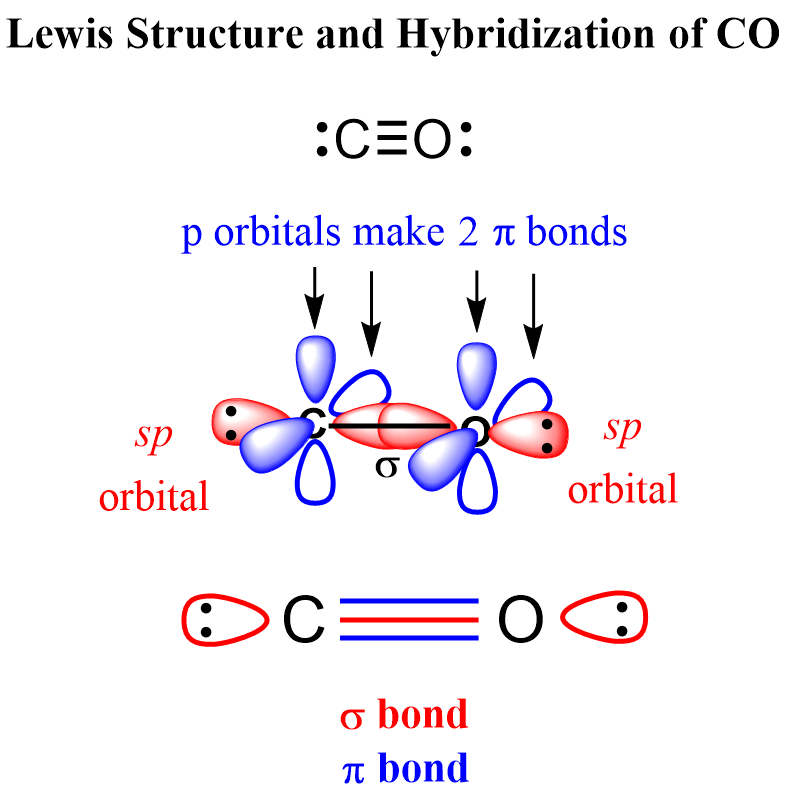
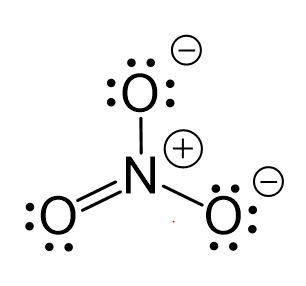
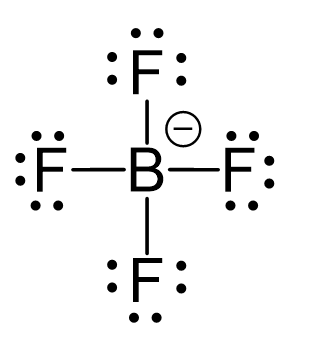
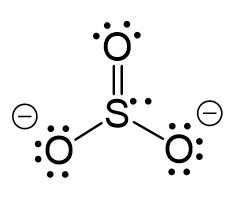
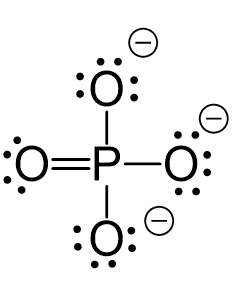
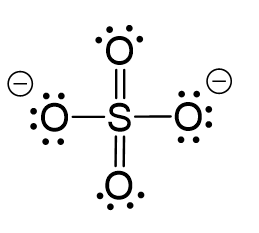
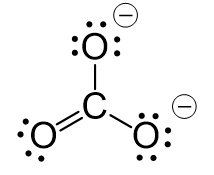
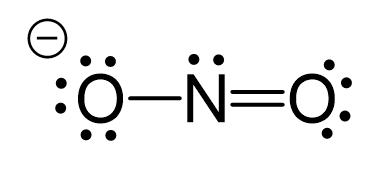CO2 Geometry and Hybridization
First, we need to draw the Lewis structure of CO2. In short, these are the steps you need to follow for drawing a Lewis structure: 1. Write the correct skeletal structure for the molecule. * Hydrogen atoms are … Read more