B is the central atom, so we can draw a preliminary skeletal structure:
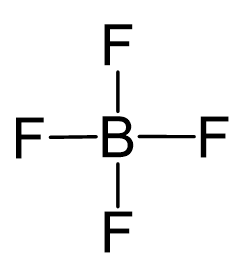
There is a total of 3 + 4×7 + 1 = 32 electrons, and 8 are used to make the covalent bonds. Halogens on terminal positions are always going to have 3 lone pairs of electrons, so 4×6 = 24 electrons go on the fluorenes. The boron has a -1 charge since it has four bonds and it is in group 3:
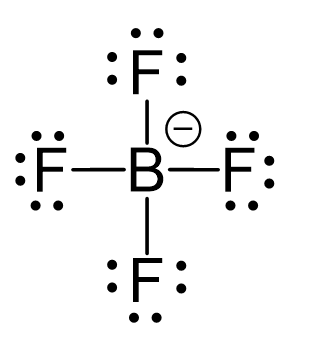
The central atom has 4 atoms and no lone pairs, therefore, both the electron and molecular geometries are tetrahedral.
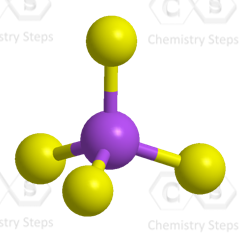
Steric number 4 corresponds to sp3-hybridization where the idealized bond angles are 109.5o.
More practice examples on geometry and hybridization in this multiple-choice quiz:
Check Also


Would anybody have an idea what the structure of [Zn(NH3)][BF4]2 would look like?
Thanks!