In this set of practice problems, we will go over the atomic structure, subatomic particles, determining the number of protons, neutrons, and electrons, as well as writing correct chemical formulas.
Check Also
Practice
All the atoms of a given element have the same ________.
a) number of neutrons
b) number of protons
c) atomic mass
d) number of electrons
e) volume
Which atom has the smallest number of protons?
a) Li
b) Mn
c) Be
d) Ag
e) As
Which atom has the largest number of protons?
a) 11B
b) 14C
c) 14N
d) 19F
e) 18O
Which combination of protons, neutrons, and electrons is correct for the 59Ni isotope?
A) 29 p+, 30 n°, 29 e-
B) 28 p+, 30 n°, 28 e-
C) 28 p+, 31 n°, 28 e-
D) 28 p+, 32 n°, 31 e-
E) 27 p+, 32 n°, 27 e-
What is the average atomic mass of the element X if it contains 92.23 % of isotope X-27.976, 4.683 % of X-28.976, and 3.087 % X-29.973?
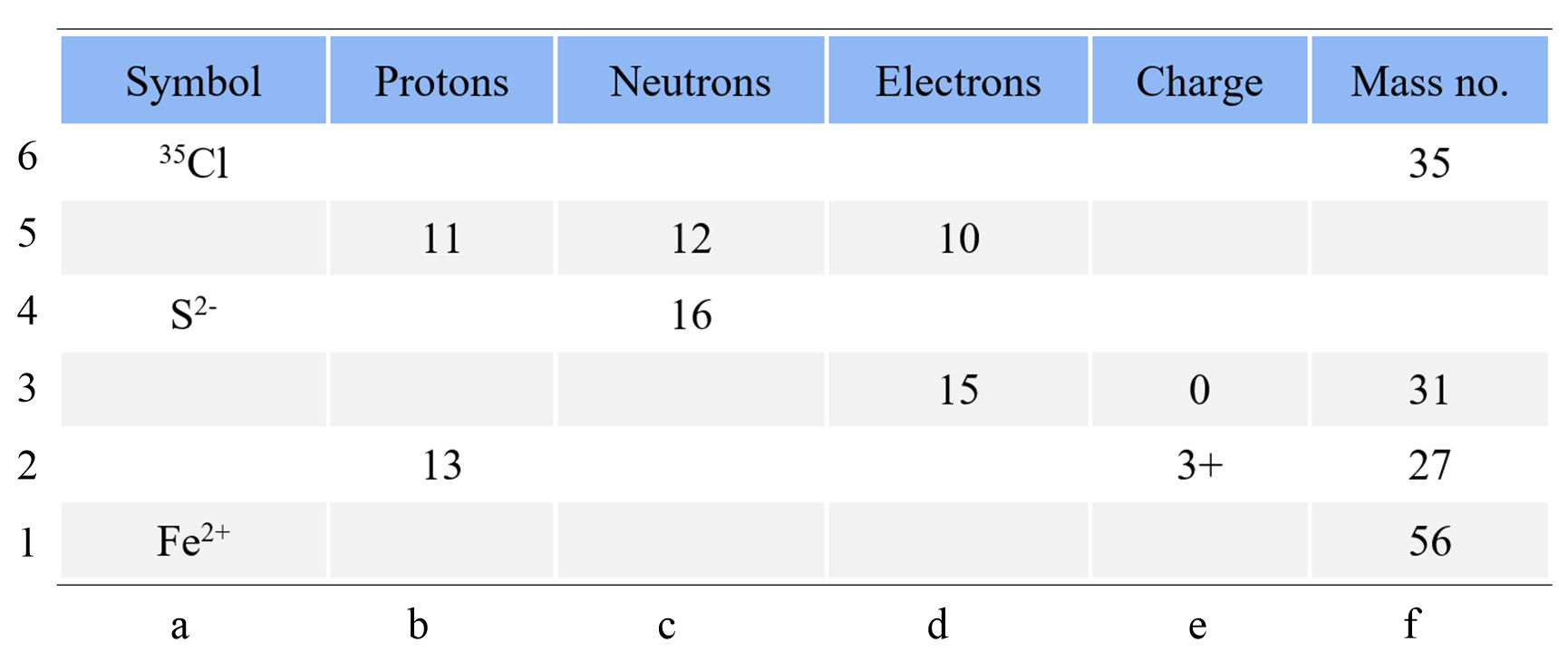
Complete the following table using the periodic table of elements. Add the specific isotope with the charge where needed.